Reagent kit and method for detecting tumor recurrence and transfer and evaluating curative effect
A technology for tumor metastasis and kits, which can be used in measurement devices, instruments, scientific instruments, etc., and can solve problems such as protein sequence identification and analysis, difficult chemical structures, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 A kit and method for detecting tumor recurrence, metastasis and evaluating curative effect
[0054] (1) Experimental method
[0055] 1. Materials
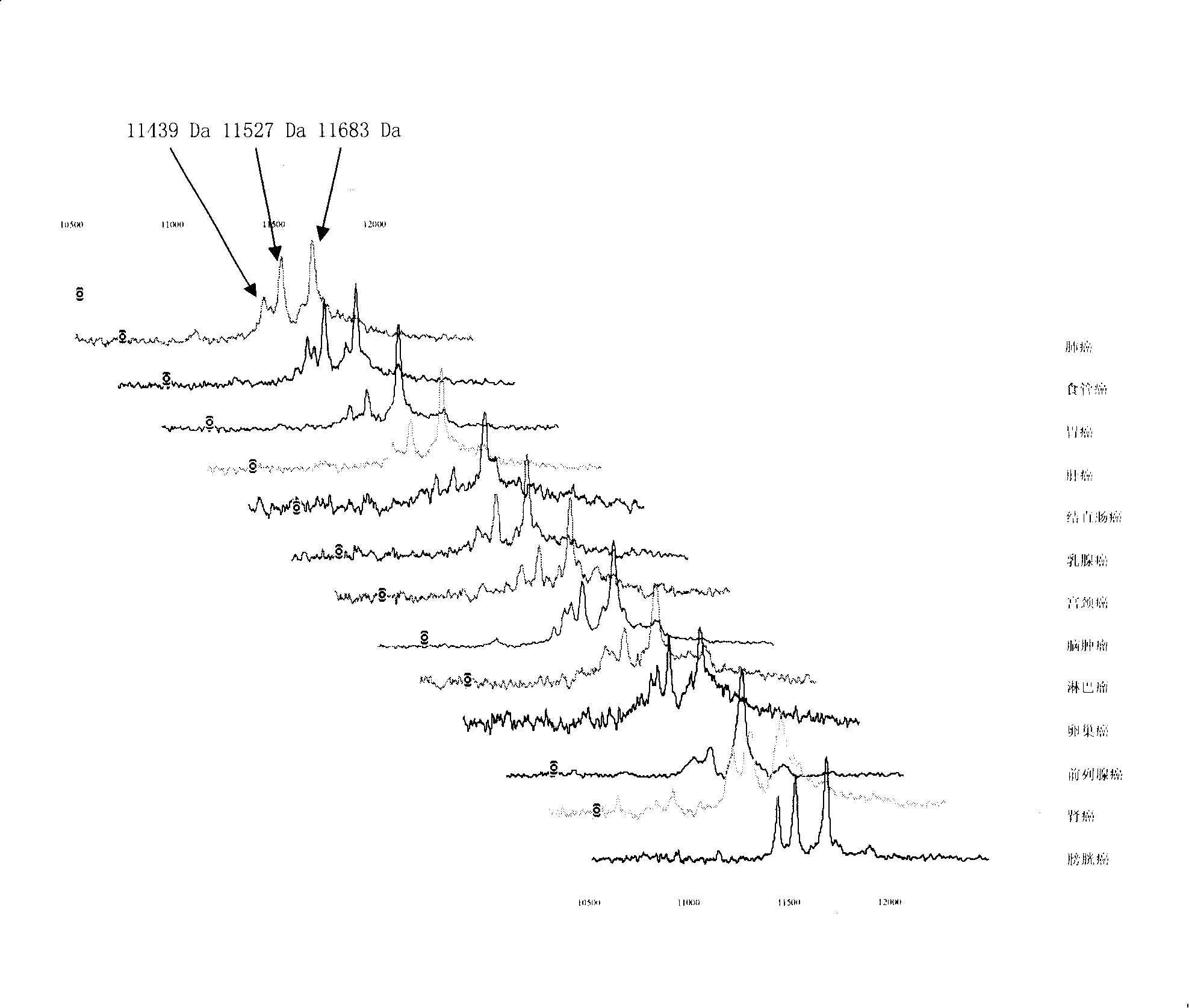
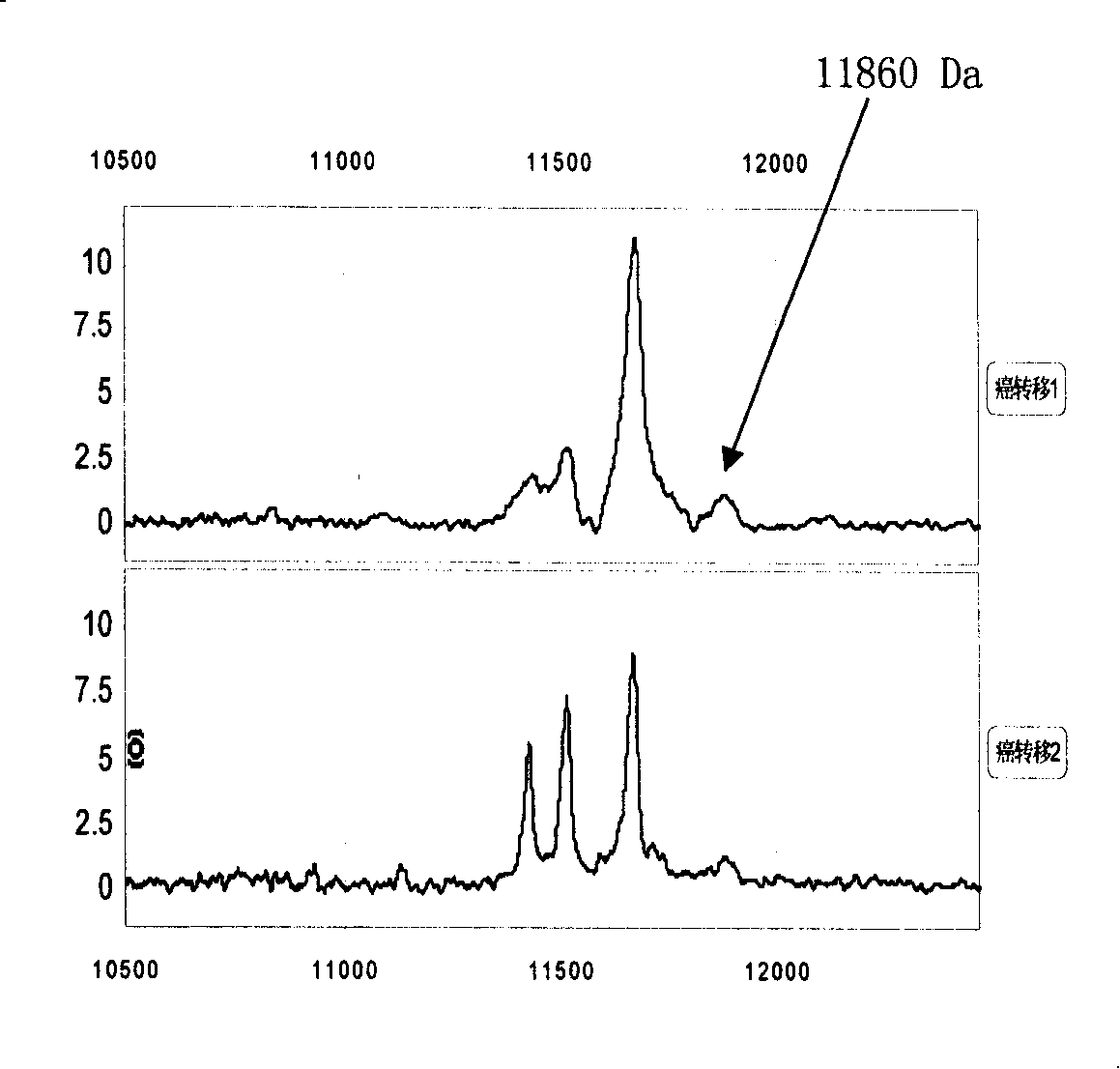
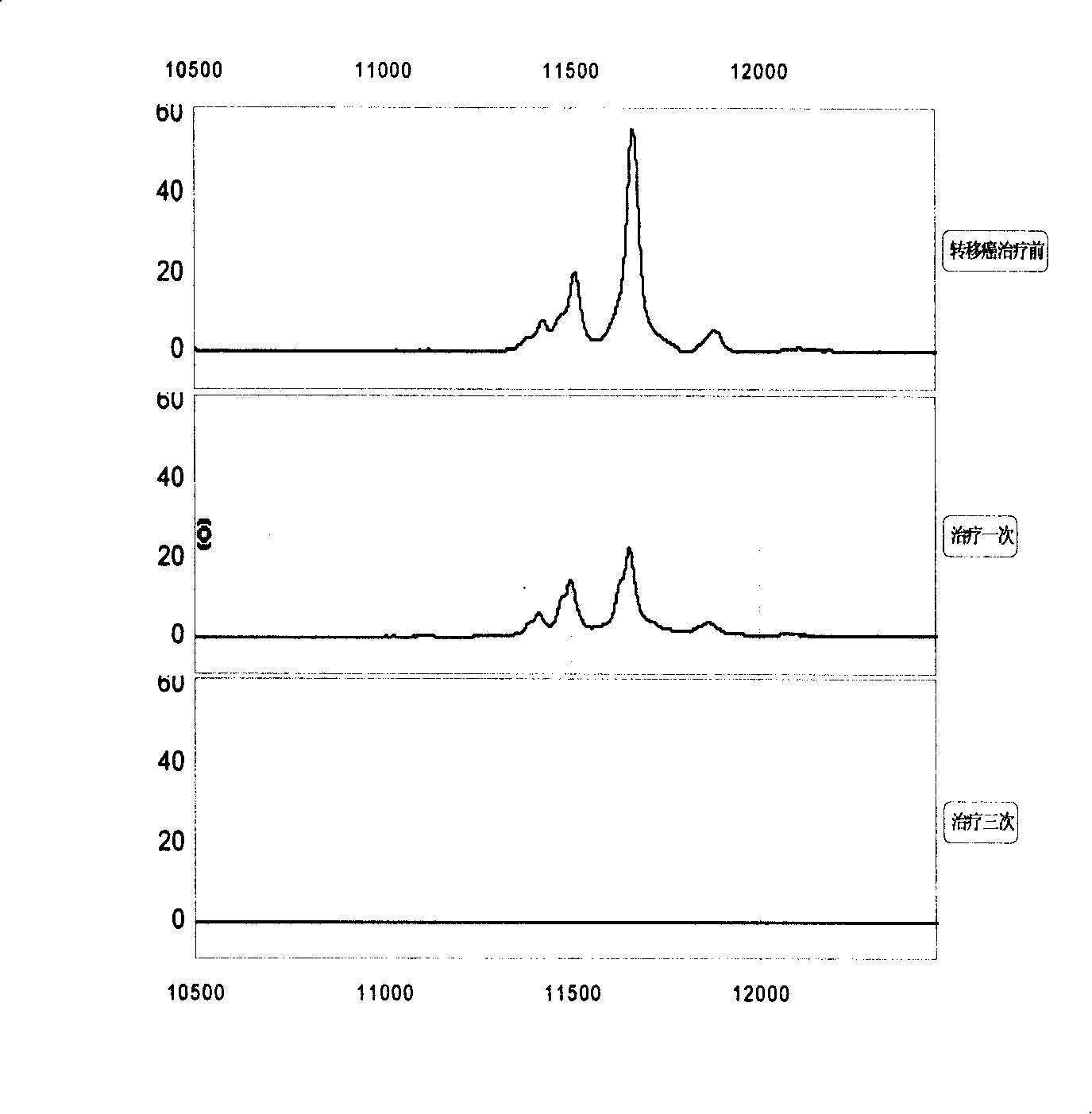
[0056] 1. Specimen sources: blood samples from lung cancer, esophageal cancer, gastric cancer, liver cancer, colon cancer, breast cancer, cervical cancer, brain tumor, lymphoma, ovarian cancer, prostate cancer, pancreatic cancer, bladder cancer, nasopharyngeal cancer, etc. (serum and plasma). A. 50 tumors no transfer Patient's serum; B. 20 tumors transfer Patient's serum; C. 30 cases of different tumors relapse Patient's serum; D. 20 patients with recurrent and metastatic tumors After effective treatment serum.
[0057] 2. Quality control: A. Human standardized quality control serum B. Mass spectrometer laser energy regulation: Before each test, use the above-mentioned standardized quality control serum.
[0058] 2. Method
[0059]1. Collection of samples: After the whole blood is collected, draw the se...
Embodiment 2
[0072] Example 2 Molecular Identification of Serum Amyloid A in Blood
[0073] Carbodiimide method (Carbodiimide Method) will have carboxylic acid group labeled magnetic bead on the matrix and the amino group of anti-serum amyloid A antibody (Gunn DL, et al.Preparation of sensitive and stable erythrocytes by the carbodiimidc method for the detection of primary and secondary IgM and IgG antibody. J Immunol Methods. 1972; 1(4): 381-389.).
[0074] Samples were spotted on the site of an anti-serum amyloid A antibody matrix.
[0075] Wash with binding buffer. Apply the first wash solution to the site before the sample is completely dry. The wash solution was left on the spot for at least 10 seconds. Thoroughly remove the first wash solution and repeat the above steps with the second wash solution. Thoroughly wash the entire array point with 1% trifluoroacetic acid, elute the biomarkers onto a special metal sheet for mass spectrometry (with 3×3mm round holes), dry the metal she...
Embodiment 3
[0084] Example 3 Sorting identification of mutated serum amyloid A
[0085] The 11439±15, 11527±15 and 11683±15 Da biomarkers were sequenced using multiple-stage mass spectrometry (MS / MS), post-source fragmentation (PSD) and protein ladder sequencing. By breaking molecules into pieces, protein ladders can be generated. This gradient is then analyzed by mass spectrometry. The 11439±15, 11527±15 and 11683±15 Da biomarkers were identified as variant serum amyloid A. Its chemical structure is (104 amino acids arranged from N-terminal to C-terminal):
[0086] Chemical structures of 11683 ± 15 Da biomarkers:
[0087] N-terminal
[0088] RSFFSFLGEAFDGARDMWRAYSDMREANYIGSDKYFHARGNYDAAKRGPGGVWAAEAISDARENIQRFFGHGAEDSLADQAANEWGRSGKDPNHFRPAGLPEKY
[0089] C-terminal.
[0090] Chemical structure of the 11527±15 Da biomarker:
[0091] N-terminal
[0092] SFFSFLGEAFDGARDMWRAYSDMREANYIGSDKYFHARGNYDAAKRGPGGVWAAEAISDARENIQRFFGHGAEDSLADQAANEWGRSGKDPNHFRPAGLPEKY
[0093] C-terminal.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com