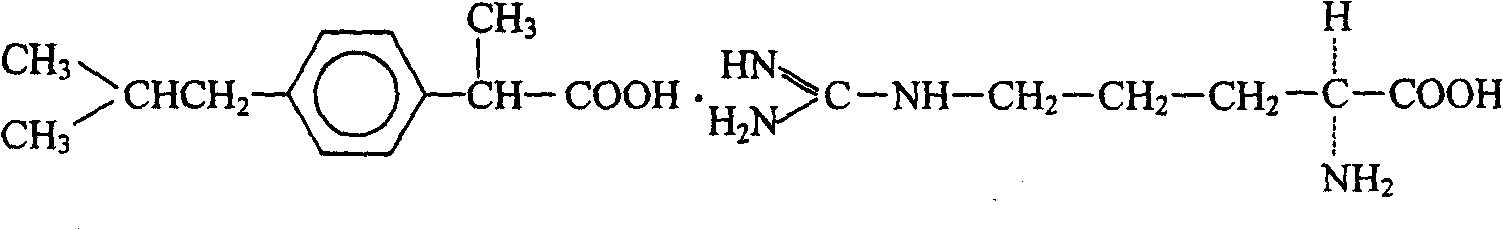
Method for preparing ibuprofen arginine
A technology of arginine ibuprofen and arginine, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of solvents that are difficult to recycle, increase the difficulty of quality control, and consume energy. Achieve the effect of reducing synthesis process steps and reaction equipment, shortening production process and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Get 41g of ibuprofen, dissolve in 230mL of 95% ethanol, get 35g of L-arginine, add to the ibuprofen ethanol solution under stirring. Heat to 70°C, stir and react for 30 minutes, then cool to crystallize at room temperature, filter with suction, dry at 50°C, and weigh to obtain 68.8g of the product. mp165-167°C.
Embodiment 2
[0022] Get 41g of ibuprofen, dissolve in 340mL of 95% ethanol, get 27.7g of L-arginine, add to the ibuprofen ethanol solution under stirring. Heat to reflux, reflux and stir for 20 minutes, then cool to crystallize at room temperature, filter with suction, dry at 50°C, and weigh to obtain 52.4 g of the product. mp165-167°C.
Embodiment 3
[0024] Get 206g of ibuprofen, be dissolved in 760mL of 95% ethanol, get 174g of L-arginine, add in the ibuprofen ethanol solution under stirring. Heat to 50°C, stir and react for 50 minutes, then cool to crystallize at room temperature, filter with suction, dry at 50°C, and weigh to obtain 330 g of the product. mp165-167°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com