Unmethylated CpG dinucleotide content detection method
A dinucleotide and methylase technology, applied in biochemical equipment and methods, microbial determination/inspection, measurement devices, etc., can solve the problems of high preparation cost, hindering clinical application and promotion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
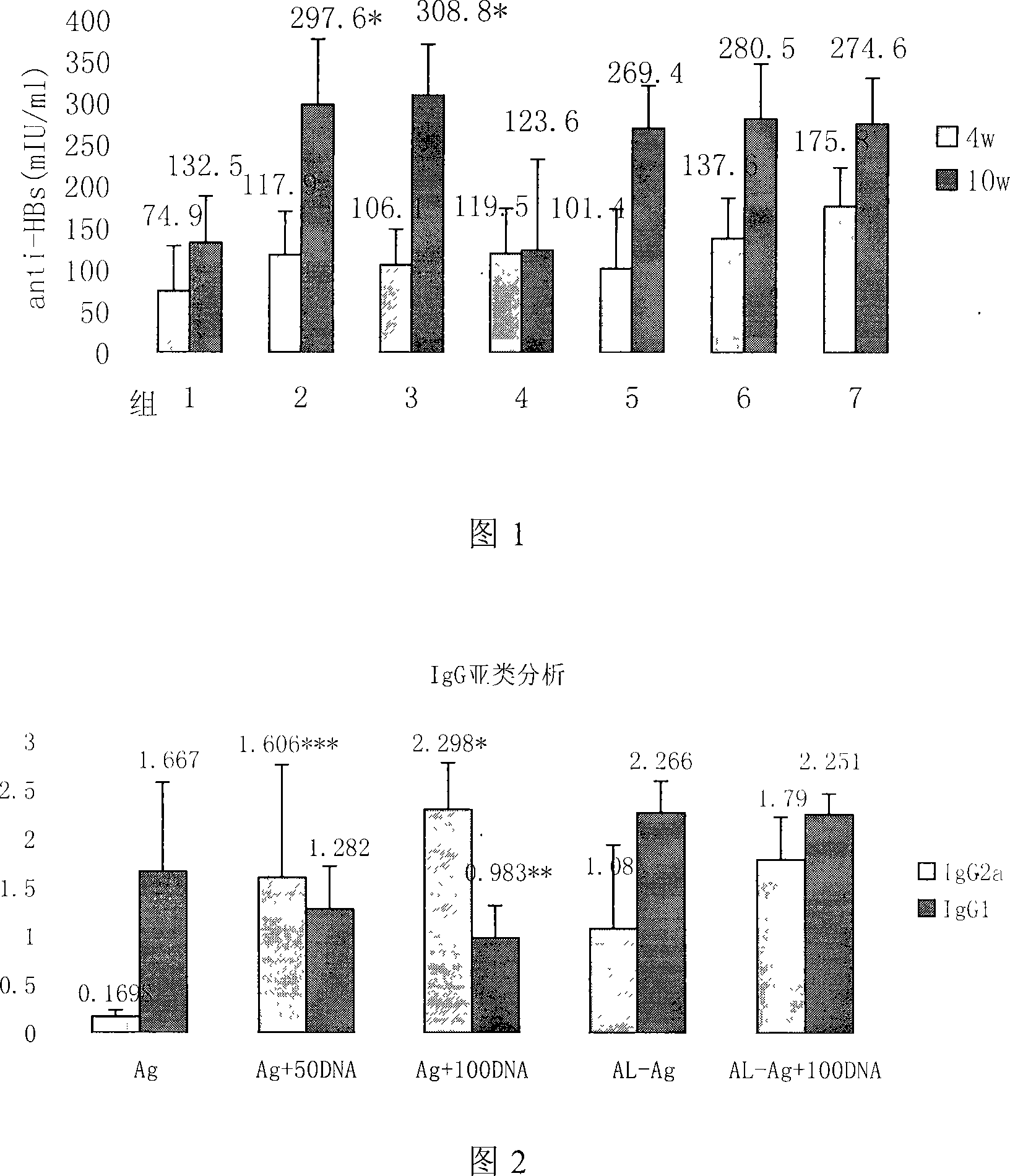
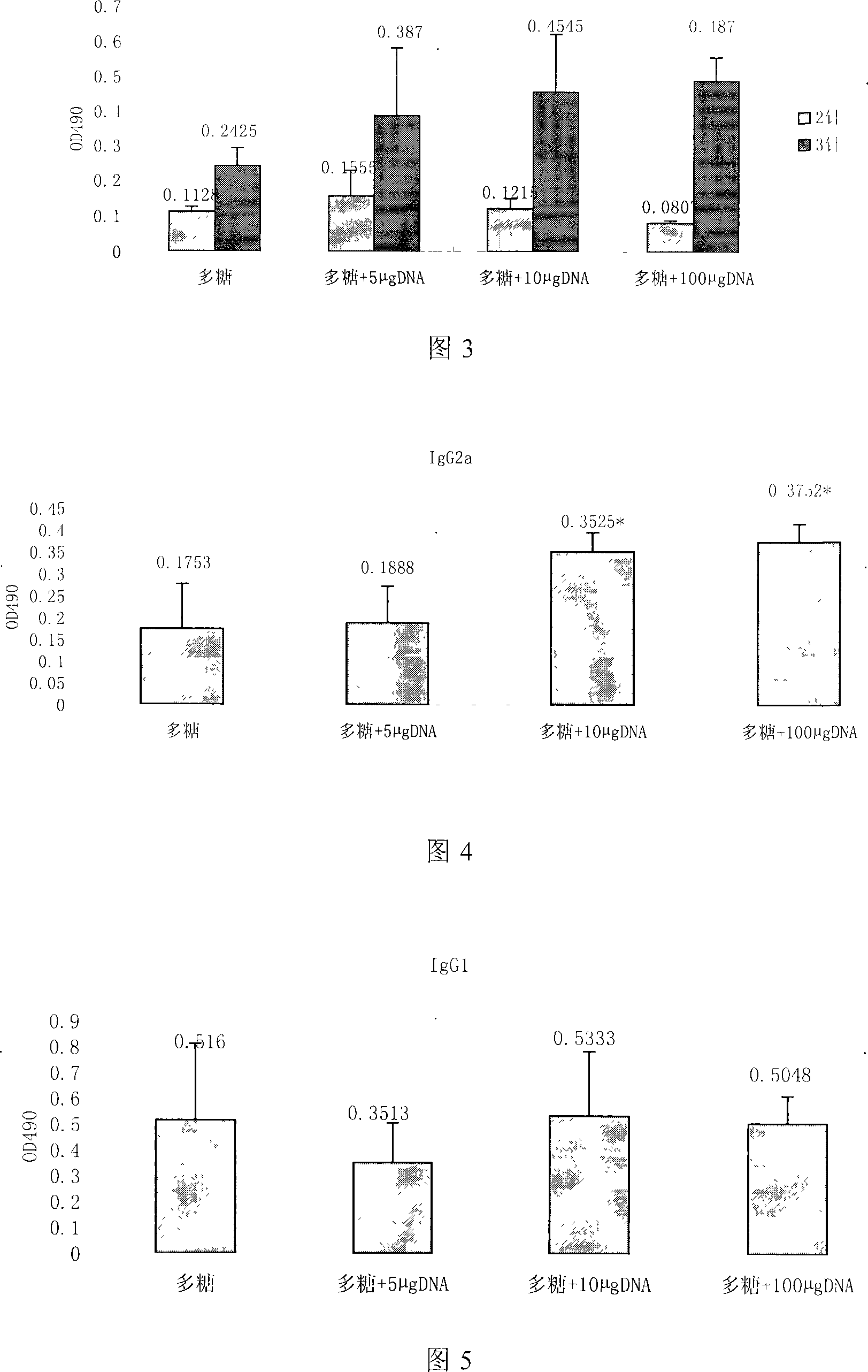
[0024] The innovation and application significance of the present invention are described in detail below through specific examples and test results to help readers better understand the spirit and essence of the present invention, but it does not constitute a limitation to the implementation scope of the present invention.
[0025] 1. Preparation of BCG-CpG-DNA
[0026] Step 1, bacterial cell culture: dissolve the strain preserved at low temperature in liquid (BCG strain D2PB302 for the preparation of BCG in China, provided by the vaccine department of the China Institute for the Control of Pharmaceutical and Biological Products) at room temperature, and inoculate it in Potato Sutong medium. After culturing at 37°C for 15 days, transfer to improved liquid Sutong medium, and culture at 37°C for 14-20 days;
[0027] in,
[0028] The preparation method of potato Sutong culture medium can be:
[0029] Take and wash fresh potatoes (1), pierce them into cylinders with a piercer, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com