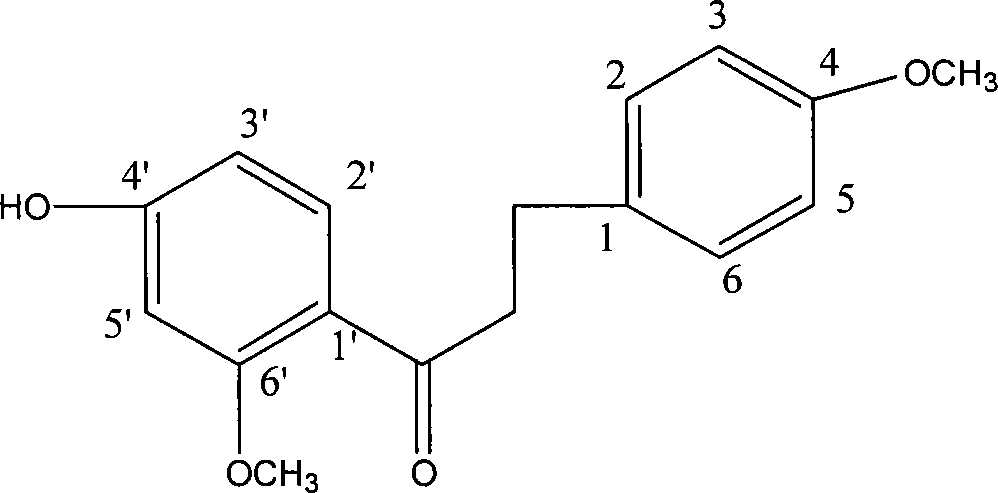
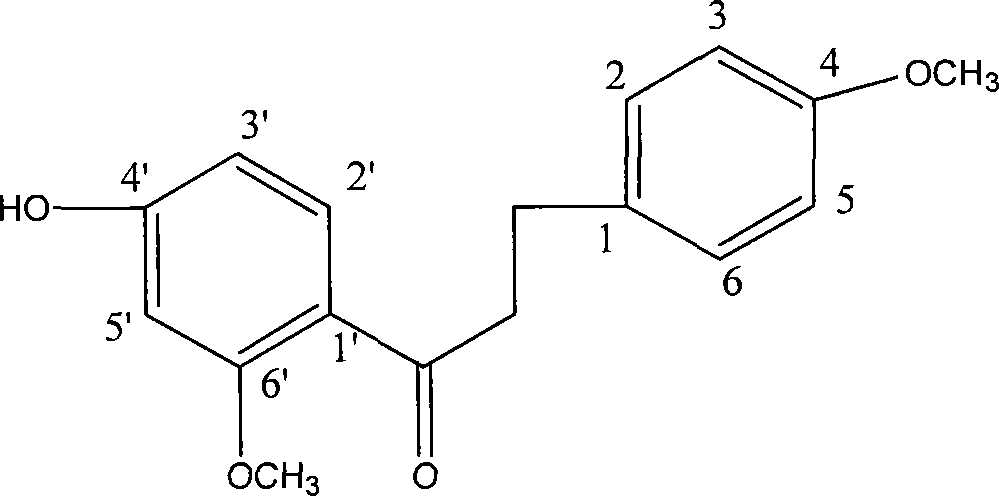
4'-hydroxy-4,6'-dimethoxy dihydrocharcone and method for synthesizing the same
A technology of dimethoxydihydrochalcone and methoxyacetophenone is applied in the field of organic chemical synthesis technology of dihydrochalcone to achieve the effects of good platelet aggregation, inhibition of platelet aggregation, and safe and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
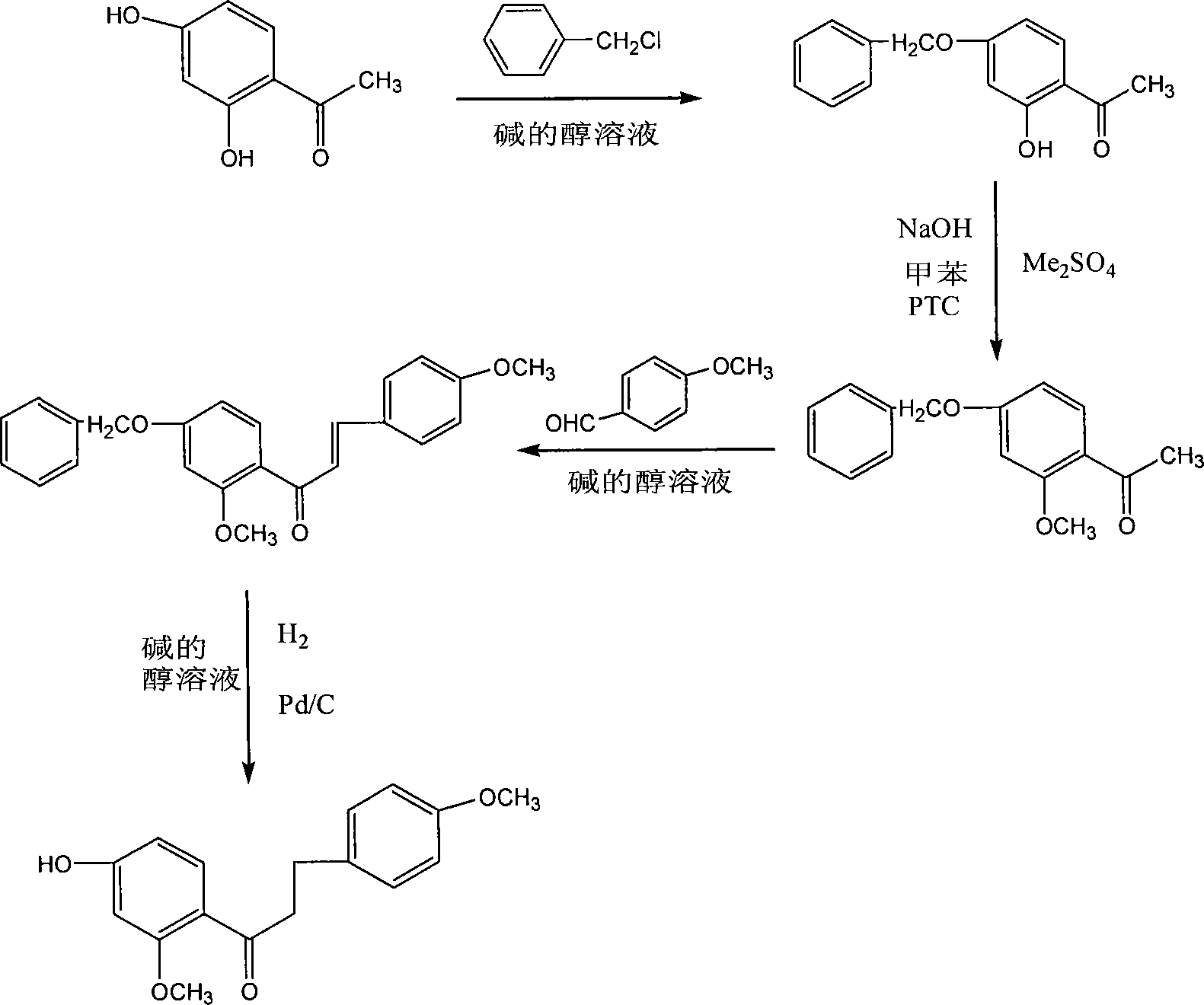
[0039] Add 2g of 2-hydroxy-4-benzyl-acetophenone (0.008mol) into a 250ml three-necked flask, and dissolve the toluene. Add 0.2 g of phase transfer catalyst benzyltriethylammonium chloride, 2 mL of dimethyl sulfate. While stirring mechanically at room temperature, 40% NaOH was slowly added dropwise to control the pH of the reaction system to be 8-9. The reaction was followed by TLC and the starting material spot disappeared in 3 hours. The organic phase was evaporated to dryness in toluene. 0.72 g of solid was obtained, which was 4-benzyloxy-2-methoxyacetophenone. Recrystallization from ethyl acetate gave 0.6 g of tan crystals. The melting point is 64.8-65.6°C. The purity detected by high performance liquid chromatography was 98.5%, and the yield was 29.3%.
[0040] in N 2 In the protected there-necked flask, add 2.8g (0.011mol) 4-benzyloxy-2-methoxyacetophenone, 1.5g (0.011mol) 4-methoxybenzaldehyde, add 100mL ethanol and 11.2g (0.2mol) KOH solution. After completion, ...
Embodiment 2
[0043] Add 2g of 2-hydroxy-4-benzyl-acetophenone (0.008mol) into a 250ml three-necked flask, and dissolve the toluene. Add 0.3 g of phase transfer catalyst benzyltriethylammonium chloride, 2.5 mL of dimethyl sulfate. Stir mechanically at room temperature while slowly adding 30% NaOH dropwise to control the pH of the reaction system to 8-9. The reaction was followed by TLC and the starting material spot disappeared in 3.5 hours. The organic phase was evaporated to dryness in toluene. 0.79 g of solid was obtained, namely 4-benzyloxy-2-methoxyacetophenone, which was recrystallized from ethyl acetate to obtain 0.7 g of brown-yellow crystals. The melting point is 64.4-65.2°C. The purity detected by high performance liquid chromatography was 98.5%, and the yield was 34.2%.
[0044] in N 2 In the protected three-necked flask, add 2.8g (0.011mol) 4-benzyloxy-2-methoxyacetophenone, 2g (0.015mol) 4-methoxybenzaldehyde, add under stirring by 150mL ethanol and 8.4 g (0.15mol) KOH so...
Embodiment 3
[0047] Add 2g of 2-hydroxy-4-benzyl-acetophenone (0.008mol) into a 250ml three-necked flask, and dissolve the toluene. Add 0.1 g of phase transfer catalyst benzyltriethylammonium chloride, 3 ml of dimethyl sulfate. While mechanically stirring at room temperature, 20% NaOH was slowly added dropwise to control the pH of the reaction system to 8-10. The reaction was followed by TLC, and the starting material spot disappeared after 5 hours. The organic phase was evaporated to dryness in toluene. 0.8 g of solid was obtained, which was 4-benzyloxy-2-methoxyacetophenone. Recrystallization from ethyl acetate gave 0.75 g of tan crystals. The melting point is 64.2-64.9°C. The purity detected by high performance liquid chromatography was 99.5%, and the yield was 36.63%.
[0048] in N 2 In the protected there-necked flask, add 2.8g (0.011mol) 4-benzyloxy-2-methoxyacetophenone, 1.89g (0.014mol) 4-methoxybenzaldehyde, add 200mL ethanol and 11.2g (0.2mol) KOH solution. After completi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com