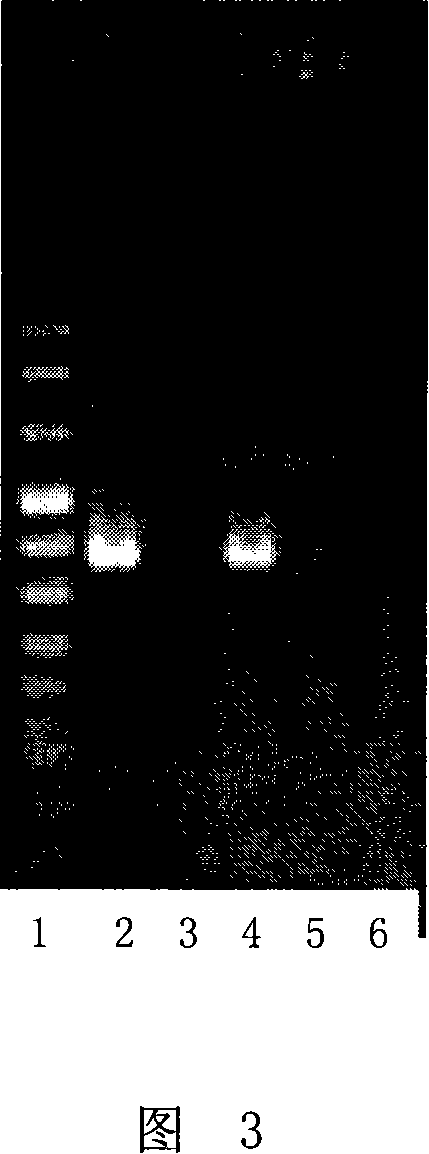
Method for detecting aphid or plant infection RhPV virus
A detection method and aphid technology, which are applied in the determination/inspection of microorganisms, biochemical equipment and methods, etc., can solve the problem of lack of rapid molecular biology detection methods, shorten the time required for detection, reduce the sample volume, and achieve accurate detection. The effect of increasing the degree of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1, the detection method of RhPV virus infection in aphids or plants of the present invention detects RhPV virus infection in aphids
[0013] 1. Extraction of aphid sample RNA: randomly collect 10 aphids from the susceptible aphid (Rhopalosiphumpadi) population (aphid population cultivated with plants carrying the RhPV virus) respectively, divide into 2 groups, and 5 of each group are used as samples to be tested. RNeasy Plant Minikit Kit (Qiagen) was used to extract total RNA from the tested samples. The specific steps are as follows: Grind the live aphids obtained from each group in 450 μl RLT buffer solution, mix them and transfer them to a QIAshredder spin column (lilac) column (Qiagen), centrifuge at 13,000 rpm, and collect the supernatant after 2 minutes , add 1 / 2 volume of ethanol (volume percentage 96-100%) in the supernatant, mix immediately, transfer to RNeasy mini column (Qiagen), centrifuge, 10,000rpm, 15s, discard the lower layer of centrifugate, ...
Embodiment 2
[0019] Embodiment 2, the detection method of aphid or plant infection RhPV virus of the present invention detects RhPV virus infection in a single aphid
[0020] Extraction of single head sample RNA: Randomly collect single head aphids from the susceptible aphid (Rhopalosiphum padi) population (aphid population cultivated with plants carrying the RhPV virus), collect 5 aphids in total and divide them into 5 groups, with 1 head in each group, Total RNA was extracted from single aphid samples using RNeasy Plant Minikit kit (Qiagen). Specifically: put each live aphid obtained in 450 μl RLT buffer to grind, mix well and transfer to QIAshredder spin column (lilac) column (Qiagen), centrifuge at 13,000 rpm, collect supernatant after 2 min solution, add 1 / 2 volume of ethanol (96-100% by volume) in the supernatant, mix immediately, transfer to RNeasy mini column (Qiagen), centrifuge, 10,000rpm, 15s, discard the lower centrifugate, Add 500μl RPE buffer to the RNeasy mini column, centr...
Embodiment 3
[0022] Embodiment 3, the detection method of aphid or plant infection RhPV virus of the present invention detects RhPV virus infection in plants
[0023] 1. Extraction of plant sample RNA: wheat plants randomly damaged by aphids, cut leaves 0.5-1cm 2 , take 3 samples (one sample for each wheat plant). Take a 0.5-1cm leaf of a wheat plant that is harmed by poisonous aphids. 2 As a positive control, a wheat leaf 0.5-1cm without poisonous aphids 2Used as a negative control. The sample to be tested, the positive control and the negative control were taken, and the total RNA of the sample to be tested was extracted using the RNeasy Plant Minikit kit (Qiagen). Specifically: grind the obtained leaves in 450 μl RLT buffer, mix well and transfer to a QIAshredder spin column (lilac) column (Qiagen), centrifuge at 13,000 rpm, collect the supernatant after 2 minutes, and add the supernatant 1 / 2 volume of ethanol (96-100% by volume), mix immediately, transfer to RNeasy mini column (Qia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com