Catalyst system used for synthesizing acetic acid and acetic anhydride and application thereof
A catalyst and acetic anhydride technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, carboxylic anhydride preparation, etc., can solve the problem of catalytic activity, unsatisfactory stability, lack of catalytic activity, There are no problems such as catalytic activity, and the catalytic process is simple and easy, the formation is simple and rapid, and the production cost is reduced.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the preparation of bonding type rhodium ion complex: the steps are as follows:
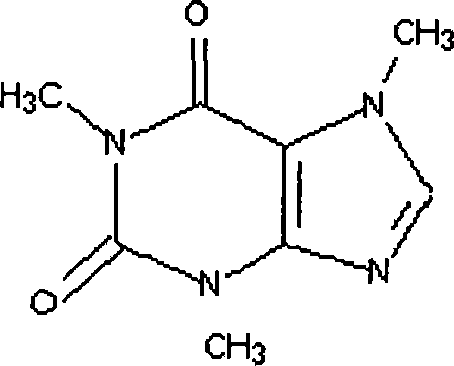
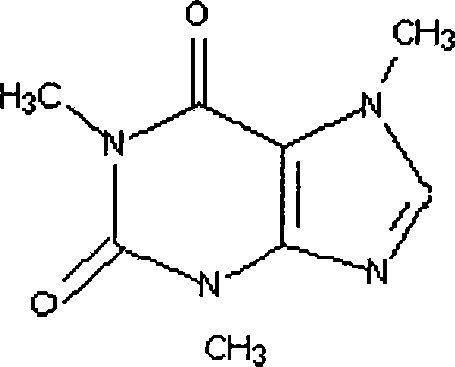
[0018] (1) Quaternization of caffeine: Add caffeine to the quaternary methyl iodide reagent, the molar ratio of caffeine and methyl iodide is 1:0.5, then add benzene to dilute, leave to react for 12 hours, and obtain part of the quaternization Ligand caffeine.
[0019] (2) Synthesis of the caffeine-bonded rhodium ion complex: the caffeine ligand after the quaternization of the above gained and dicarbonyl diiodide dirhodium [Rh 2 (CO) 2 I 2 ] Proportioning according to the mass ratio of 10:1, adding to benzene, stirring and reacting for 4 hours at room temperature and under the protection of argon, naturally drying, and drying under reduced pressure to obtain the product, which is a brown-yellow crystalline powder.
Embodiment 2
[0020] Embodiment 2: the preparation of bonding type rhodium ion complex: the steps are as follows:
[0021] (1) Quaternization of caffeine: Add caffeine to the quaternary methyl iodide reagent, the molar ratio of caffeine and methyl iodide is 1:0.8, then add ethanol to dilute, leave to react for 14 hours, and obtain part of the quaternization Ligand caffeine.
[0022] (2) Synthesis of the caffeine-bonded rhodium ion complex: the caffeine ligand after the quaternization of the above gained and dicarbonyl dichlorodirhodium [Rh 2 (CO) 2 Cl 2 ] Proportioning according to the ratio of mass ratio 20:1, adding it to ethanol, stirring and reacting for 5 hours at room temperature and under the protection of argon, drying naturally, and drying under reduced pressure to obtain the product, which is a yellow crystalline powder.
Embodiment 3
[0023] Embodiment 3: in 250ml zirconium autoclave, add dicarbonyl dichlorodirhodium [Rh 2 (CO) 2 Cl 2 ] Catalyst 0.144g, caffeine 1.0g, methyl acetate 111g, methyl iodide 31.24g, acetic acid 12g, acetic anhydride 15.3g and lithium acetate 3.9g, fill CO to remove the air in the kettle, simultaneously feed into the system H 2 Heat up to 140°C, by volume H 2 :CO=1:30, keep the kettle pressure at 4.0Mpa, stir at 500 rpm, react for 20min, the increment of acetic anhydride is 114.8g, and the space-time yield of acetic anhydride is 13.5mol / L-h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com