Novel microbial enzymes and their use
一种引物对、酪氨酸酶的技术,应用在新的真菌酶蛋白及其应用领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Plate screening of tyrosinase positive microorganisms
[0070] Indicators for tyrosinase activity screening were selected according to the literature. L-tyrosine, p-cresol, p-coumaric acid, tyramine, 3-hydroxy-2-aminobenzoic acid, and catechin were used at the concentrations indicated in Table 1. Trichoderma reesei was grown on wort agar containing selected indicators (Table 1) (Difco) for 48 days at 37°C. Visually inspect the plate for possible color changes.
[0071] T. reesei showed clear positive reactions with L-tyrosine, tyramine, 3-hydroxy-2-aminobenzoic acid and catechins. The results clearly showed that T. reesei was a tyrosinase-positive microorganism.
[0072] Table 1. Plate test screening of tyrosinase activity from T. reesei
[0073] indicator
Embodiment 2
[0075] Isolation of tyr1 and tyr2 genes from Trichoderma reesei
[0076] Two novel tyrosinase genes were amplified from genomic T. reesei DNA by PCR. Primers for tyr1 are forward:
[0077] GCT ACC GCG GAT GGG CTT CCT CGC TCG CCT CAC (SEQ ID NO: 5)
[0078] and the reverse:
[0079] CTG AGG ATC CTC AGT GGT GGT GGT GGT GGT GCT CCC ACA ACA CCAATC TCA GCA T (SEQ ID NO: 6).
[0080] Use forward primer:
[0081] GGG GAC AAG TTT GTA CAA AAA AGC AGG CTA TCA TGC TGT TGT CAGGTC CCT CTC G (SEQ ID NO: 7) and reverse primer:
[0082] GGG GAC CAC TTT GTA CAA GAA AGC TGG GTC AGT GGT GGT GGT GGTGGT GCA GAG GAG GGA TAT GGG GAA CGG CAA A (SEQ ID NO: 8) amplifies the tyr2 gene.
[0083]PCR reactions were performed with Dynazyme EXT thermostable polymerase (Finnzymes, Finland) in the reaction mixture recommended by the manufacturer. The PCR program had an initial denaturation step of 3 min at 94 °C, followed by 25 cycles of 30 s at 94 °C, 45 s at 52 °C and 2.5 min at 72 °C. This was follow...
Embodiment 3
[0085] Overexpression of the tyr2 gene in Trichoderma reesei under a strong promoter
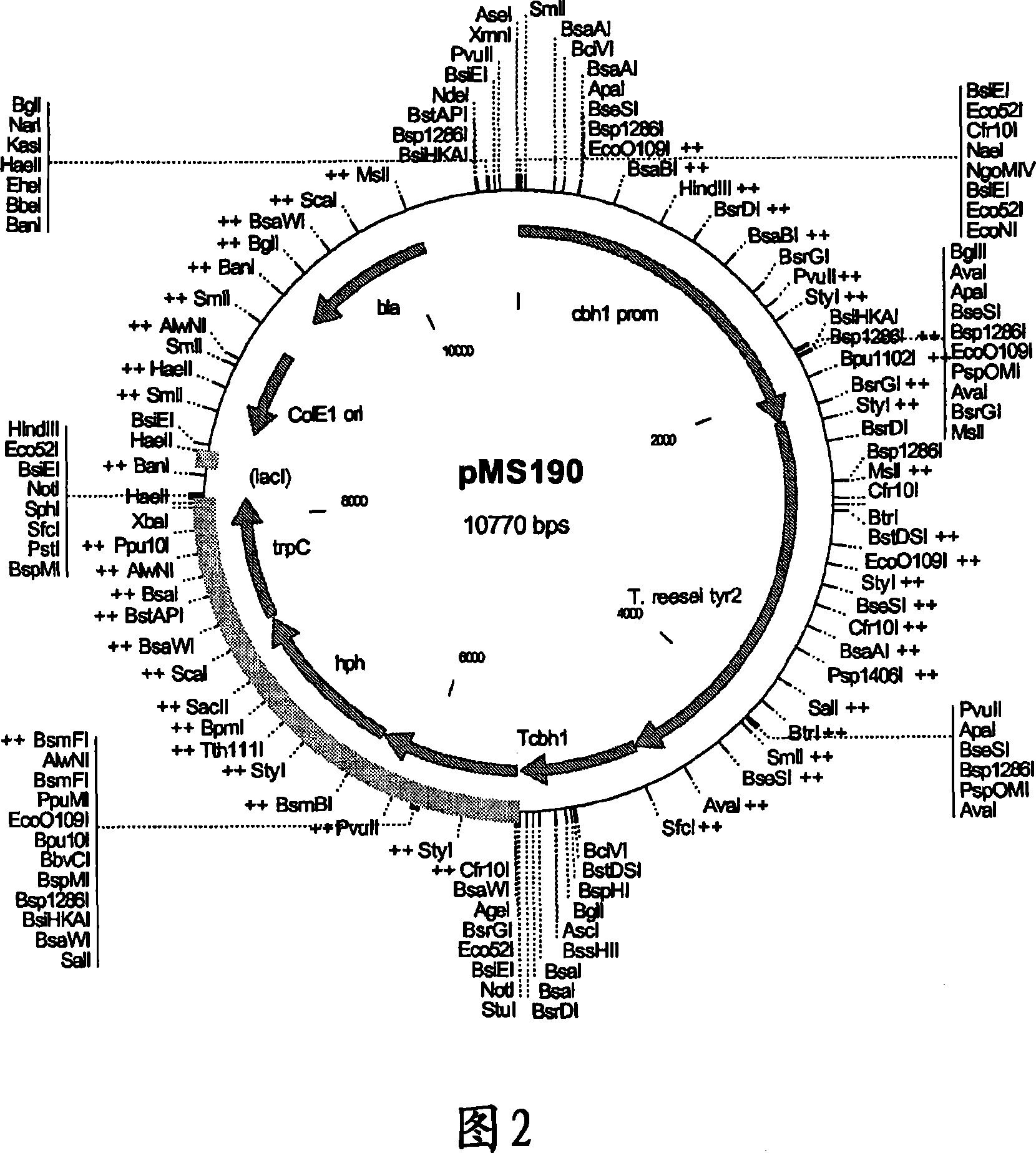
[0086] The tyr2 gene fragment was transferred from pDONR221 vector to T. reesei expression vector pMS186 by LR recombination reaction. This vector contains the Gateway reading frame cassette C (RfC) inserted between the cbhl (cellobiohydrolase 1) promoter and terminator. This vector also has a hygromycin resistance cassette for selection of T. reesei transformants. LR recombination reactions were performed using the Gateway recombination kit (Invitrogen) as directed by the manufacturer. During this recombination process, the tyr2 gene fragment was inserted between the cbh1 promoter and terminator, thereby obtaining plasmid pMS190 (Fig. 2).
[0087] Plasmid pMS190 was transformed into T. reesei strain VTT-D-00775 essentially as described (Penttil et al., 1987) and hygromycin resistant transformants were selected on plates containing 125 μg / ml hygromycin B. Transformants were streaked three...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com