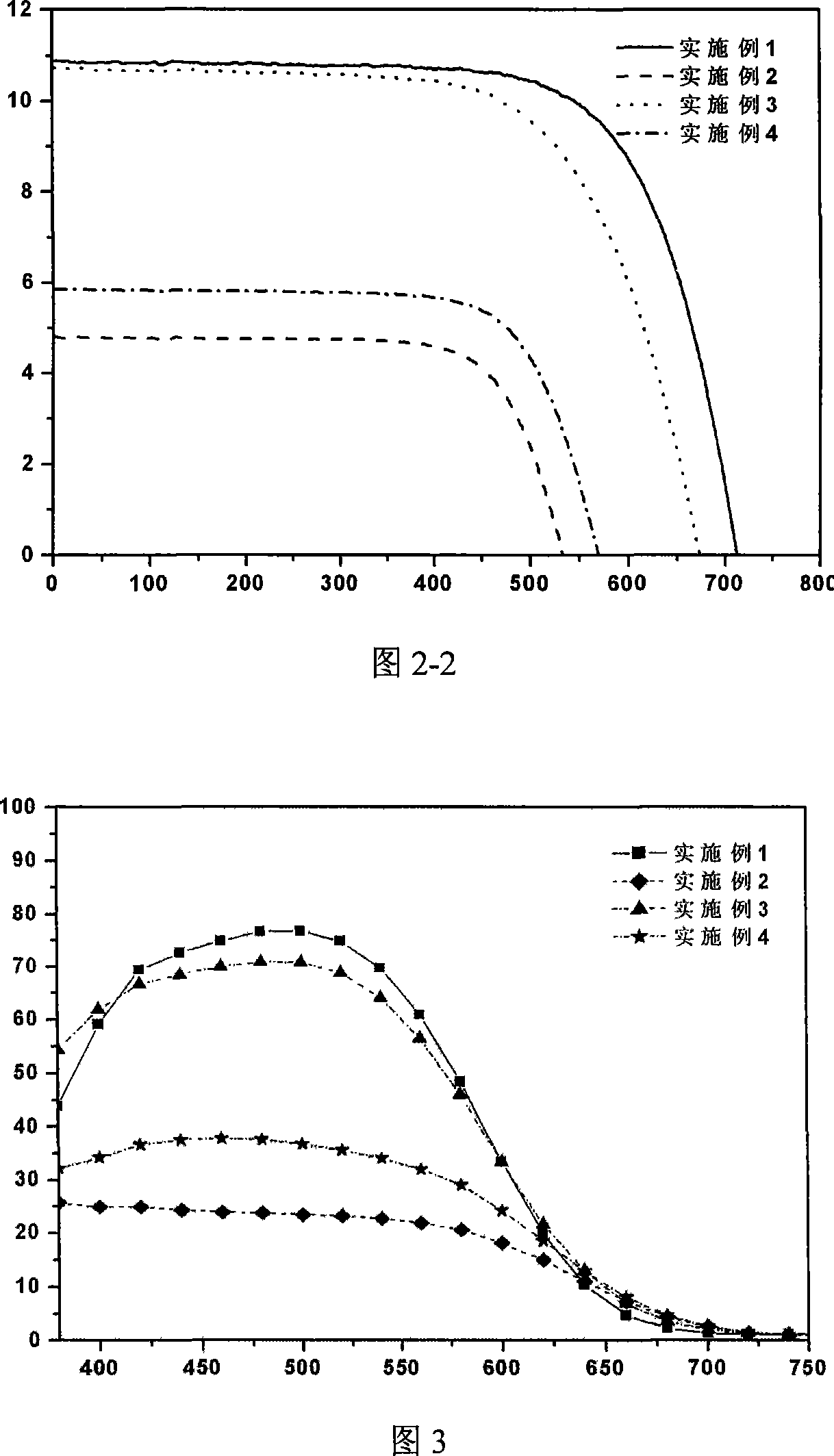
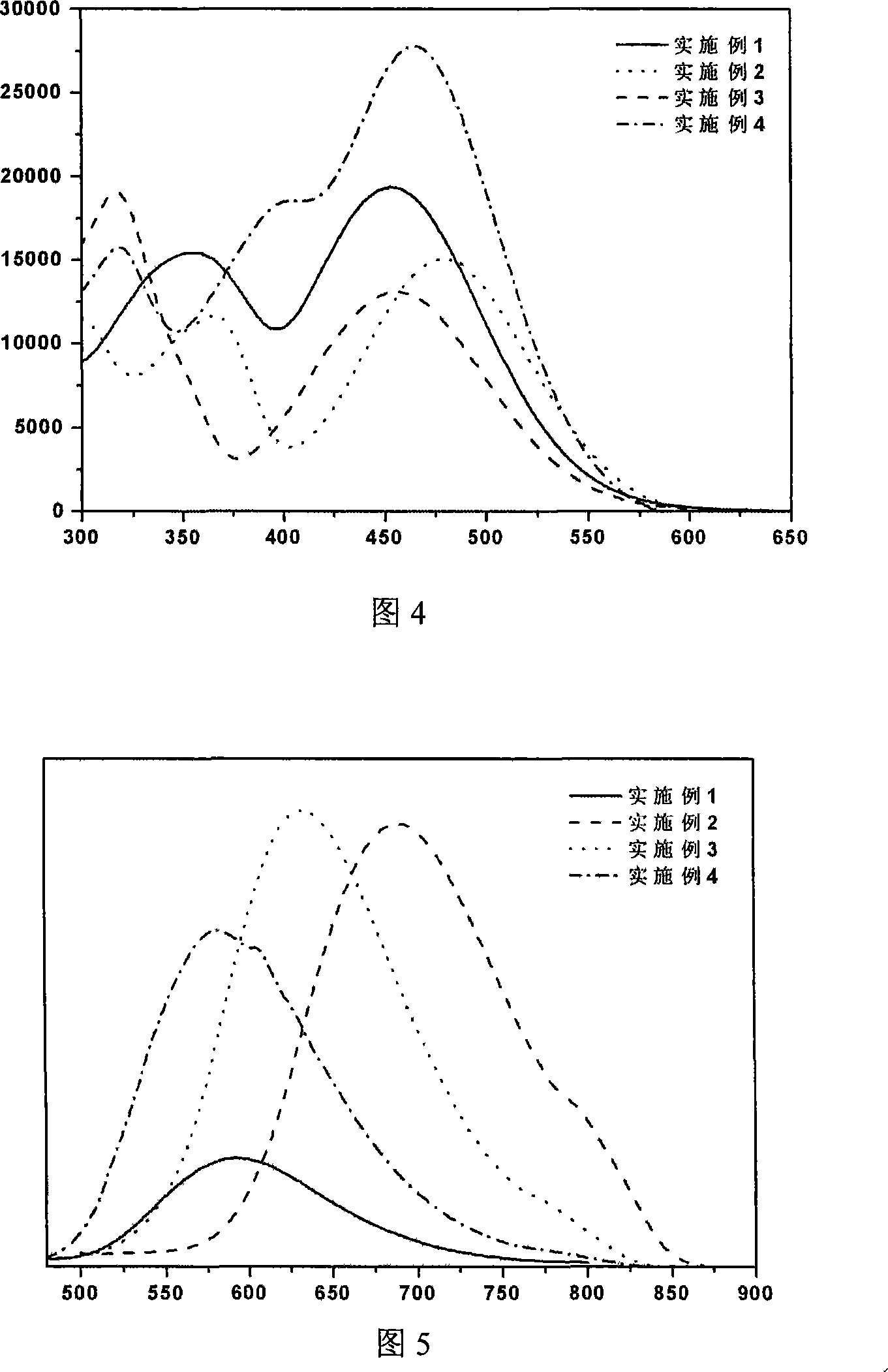
Phenothiazines dye used for dye sensitization solar battery
A dye sensitization and phenothiazine technology, applied in thiazine dyes, organic dyes, circuits, etc., can solve the problems of low color intensity, high cost, and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of N-butyl-3-cyanoacrylate-phenothiazine
[0038] All reactions were carried out in a dry environment.
[0039] (1) Synthesis of N-butyl-3-formyl-phenothiazine
[0040]
[0041] Dissolve 1.0g (3.92mmol) of N-butyl-phenothiazine in 15ml of chloroform, add 548mg of DMF (7.51mmol), stir vigorously at room temperature, and slowly add 2.3g of POCl 3 (15 mmol). Then heat up to reflux state and keep warm for 24 hours. After the reaction, the acetone was evaporated as much as possible by a rotary evaporator. Using dichloromethane as eluent to carry out column separation and purification on a silica gel column to obtain 900 mg (3.18 mmol) of compound N-butyl-3-formyl-phenothiazine with a yield of 81.1%. NMR 1 H-NMR (400MHz, Acetone-d6): δ (ppm): 0.87 (3H, t), 1.42-1.48 (2H, m), 1.73-1.80 (2H, m), 4.01 (2H, t), 6.90 ( 1H, t), 7.07 (1H, d, J = 8.0Hz), 7.13 (2H, m), 7.21 (1H, t), 7.57 (1H, d, J = 1.9Hz), 7.70 (1H, ddJ 1 =1.9Hz,J 2 =1.9Hz), 9.79(1H, s). Mass Spe...
Embodiment 2
[0046] Synthesis of N-butyl-3-(5-methylenerhodenine)-phenothiazine
[0047]
[0048] Add 120mg (0.42mmol) N-butyl-3-formyl-phenothiazine and 90mg (0.51mmol) rhodanine to 20ml acetic acid, and reflux for 3h in the presence of 50mg (0.65mmol) ammonium acetate. After cooling down to room temperature, it was poured into 50 ml of ice water, and the solid was collected by filtration. Use dichloromethane:methanol=7:1 (volume ratio) solution as the eluent to carry out column separation and purification on the silica gel column to obtain the compound N-butyl-3-(5-methylene rhodanine acid group) -Phenothiazine 140 mg (0.32 mmol), yield 76.2%. NMR 1 H-NMR (400MHz, Acetone-d6): 0.90 (3H, t), 1.43-1.49 (2H, m), 1.76-1.79 (2H, m), 4.00 (2H, t), 4.84 (2H, s), 6.97(1H, t), 7.07(1H, d, J=8.1Hz), 7.13(1H, d, J=7.6Hz), 7.17(1H, d, J=8.0Hz), 7.20(1H, t), 7.85(1H,d,J=2.0Hz), 7.47(1H,dd,J 1 =2.1Hz,J 2 =2.1Hz), 7.68 (1H, s). Mass Spectrometry TOF MS ES + : Found m / z 456.0632.Calc.for C 22...
Embodiment 3
[0050] Synthesis of N-butyl-3-[4-(2-cyanoacrylate)phenyl-1-vinyl]-phenothiazine
[0051] All reactions were carried out in a dry environment.
[0052] (1) Synthesis of N-butyl-3-hydroxymethyl-phenothiazine
[0053]
[0054] Add 81mg (2.13mmol) of sodium borocyanide to a mixed solvent of 10ml of ethanol and 10ml of dichloromethane, then add 600mg (2.12mmol) of N-butyl-3-formyl-phenothiazine in one go, at room temperature Stir for 1h. After the reaction was completed, 20ml of water was added and stirred vigorously, then extracted 3 times with dichloromethane, the collected organic phase was dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation to obtain 592mg of the compound N-butyl-3-hydroxymethyl-phenothiazine (2.07 mmol), yield 97.6%. NMR 1 H-NMR (400MHz, Acetone-d6): δ (ppm): 0.87 (3H, t), 1.40-1.45 (2H, m), 1.70-1.74 (2H, m), 3.87-3.91 (2H, t), 4.10(1H, t), 4.49(2H, d, J=5.8Hz), 6.88(1H, t), 6.93(1H, d, J=8.3Hz), 6.97(1H, d, J=8.2Hz)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com