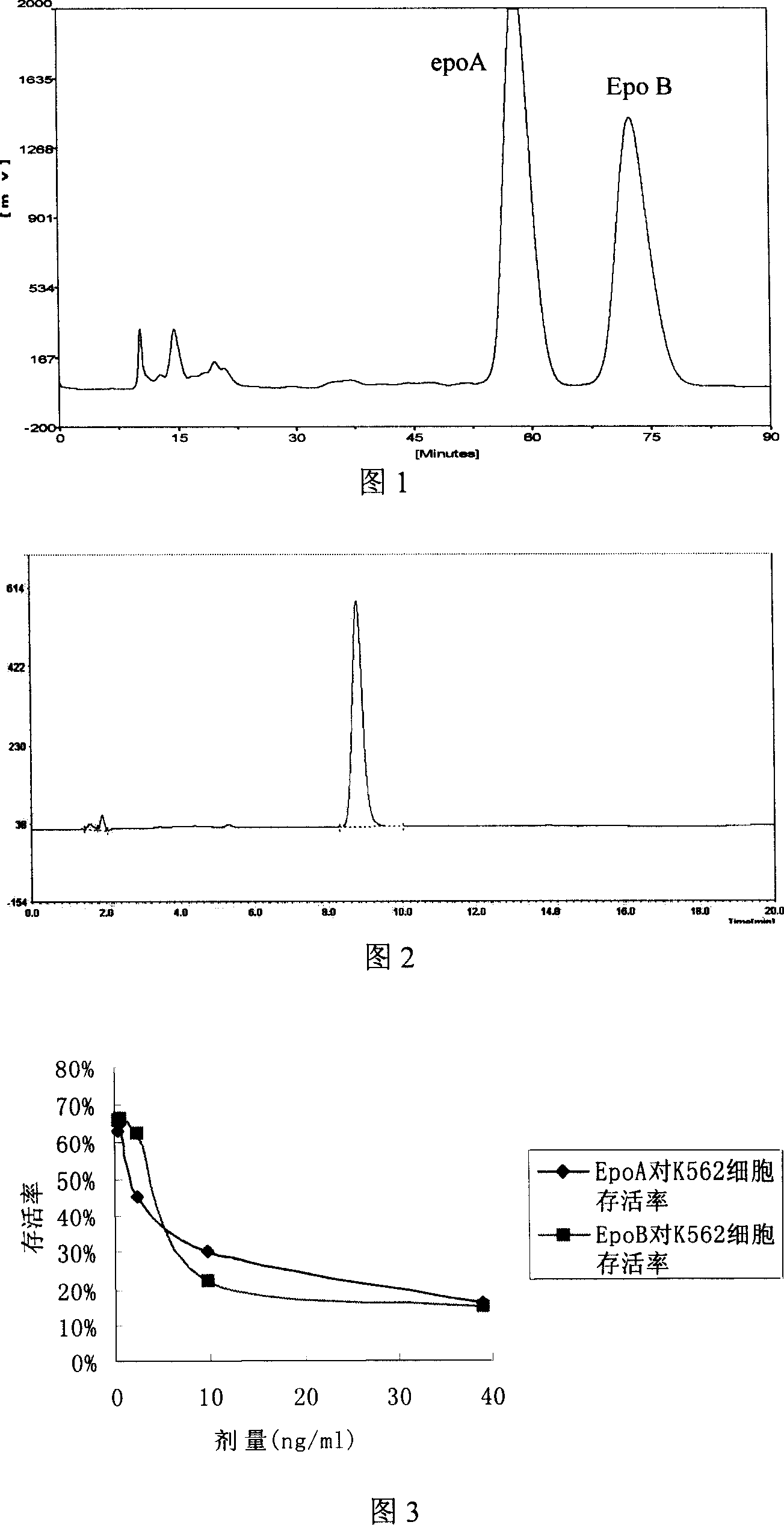
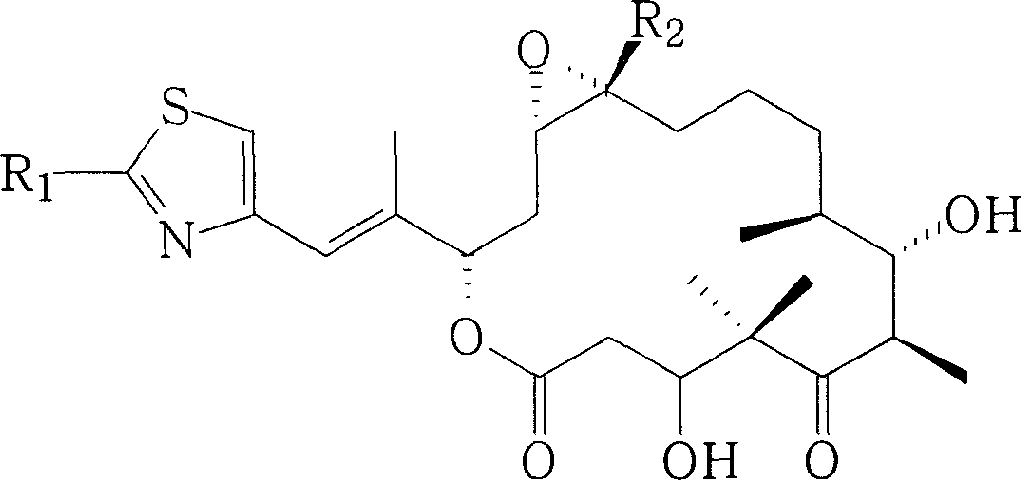
Method for preparing epothilones B and use thereof
A technology of epothilone and adsorbent, which is applied in the field of chronic myeloid leukemia drugs, can solve problems such as complex drug resistance mechanisms, and achieve the effects of easy large-scale production, strong applicability, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment one seed liquid culture
[0038] Take the frozen strains and transfer them to 50ml medium, the medium components are potato starch 8g / L, glucose 2g / L, soybean powder 2g / L, yeast extract 2g / L, etc. After culturing for 24 hours, transfer to a plurality of 500ml No. I culture medium, cultivate the seed solution for 72 hours, and transfer to a fermenter for fermentation.
Embodiment 2
[0039] Embodiment two fermentation, extract crude extract
[0040] 1) Add 65L medium to a 100L fermenter, and the medium components are potato starch 1g / L, glucose 10g / L, soybean powder 30g / L, yeast extract 15g / L, etc. Sterilize at 121°C for 20 minutes. Transplant under aseptic conditions. During the fermentation process, set the ventilation rate to 25L / min, the pressure to 0.4Mpa, the rotational speed to 100r / min, the temperature to 32°C, and the pH to 7.4. After culturing for 12 hours, 1% of the total volume of AB-8 macroporous adsorption resin was added. Harvest after culturing for 100 h.
[0041] Filter the fermented liquid with a thick cloth to obtain the adsorbent AB-8, and extract it twice with 2 times the volume of isopropanol for 1 hour each time. The isopropanol solutions were combined, concentrated to 1 / 10 by distillation under reduced pressure, and the concentrated isopropanol was extracted three times with ethyl acetate to obtain a crude epothilone B extract. ...
Embodiment 3
[0048] Embodiment three: Separation and purification of epothilone B
[0049] The crude extract containing epothilone B is separated and purified by two steps to obtain epothilone B with a purity higher than 95%.
[0050] The first step: Sephadex LH-20 column chromatography
[0051] After the extract was dissolved in methanol, the supernatant was collected by centrifugation and added to the top of Sephadex LH-20 column. Mobile phase: methanol, the mobile phase flow rate is 10cm / h. Analysis by HPLC high performance liquid chromatography (filler: Kromasil C 18 5μm, size: 4.6mm×150mm. Mobile phase: methanol: water = 7:3. Detection wavelength: UV230nm). Fractions containing epothilone B were combined for further purification.
[0052] The second step: C18 reversed-phase chromatographic separation
[0053] The fraction separated by Sephadex LH-20 was further purified by C18 reverse phase chromatography column. Mobile phase: methanol: water = 70:30, detection wavelength: UV...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com