Novel hydrochloric acid tramadol sustained-release tablet and preparation method
A technology of tramadol hydrochloride and slow-release tablets, which is applied in the field of medicine, can solve the problem of stable effective blood drug concentration not changing, and achieve the effects of maintaining blood drug concentration, increasing bioavailability, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
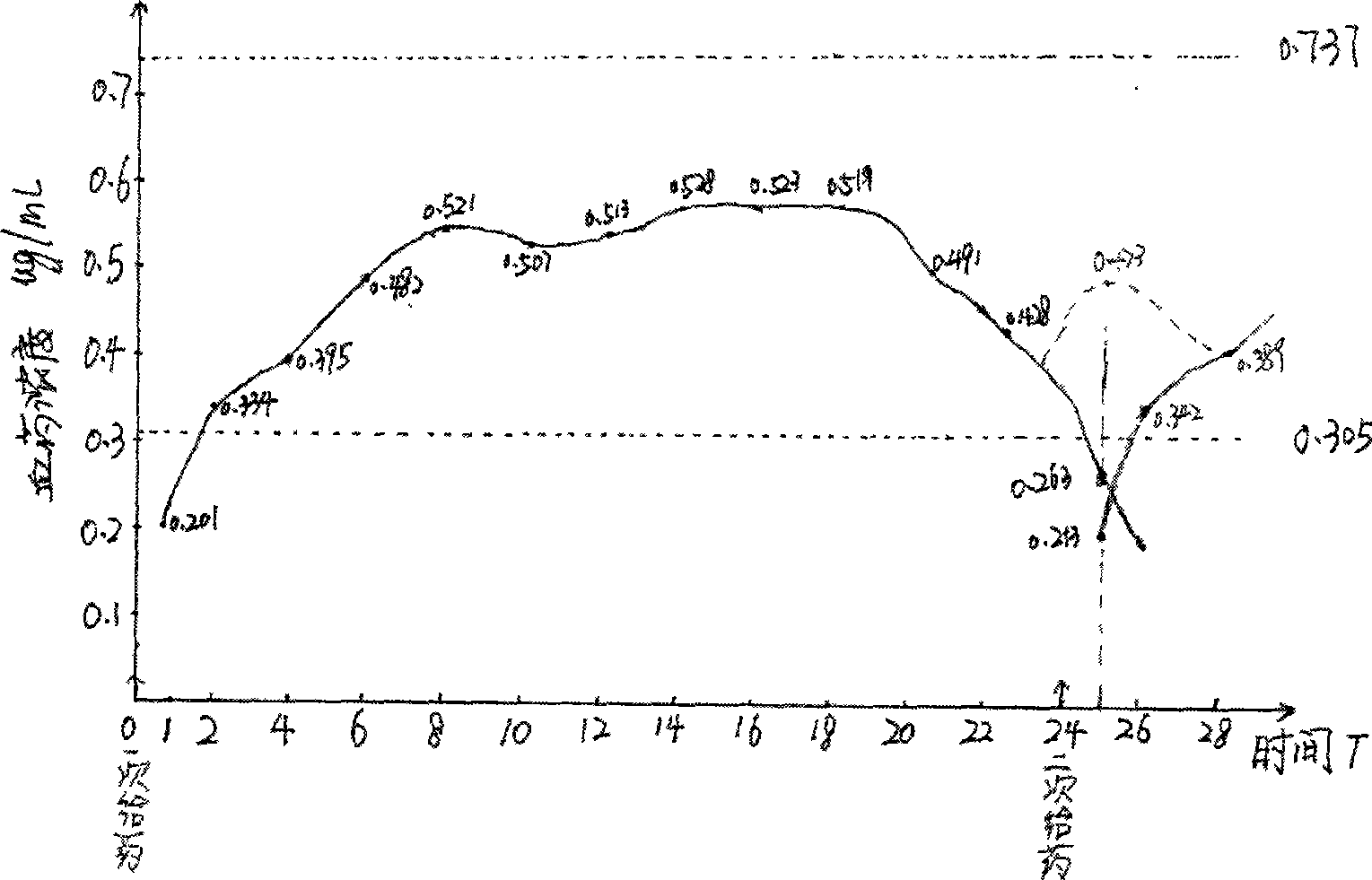
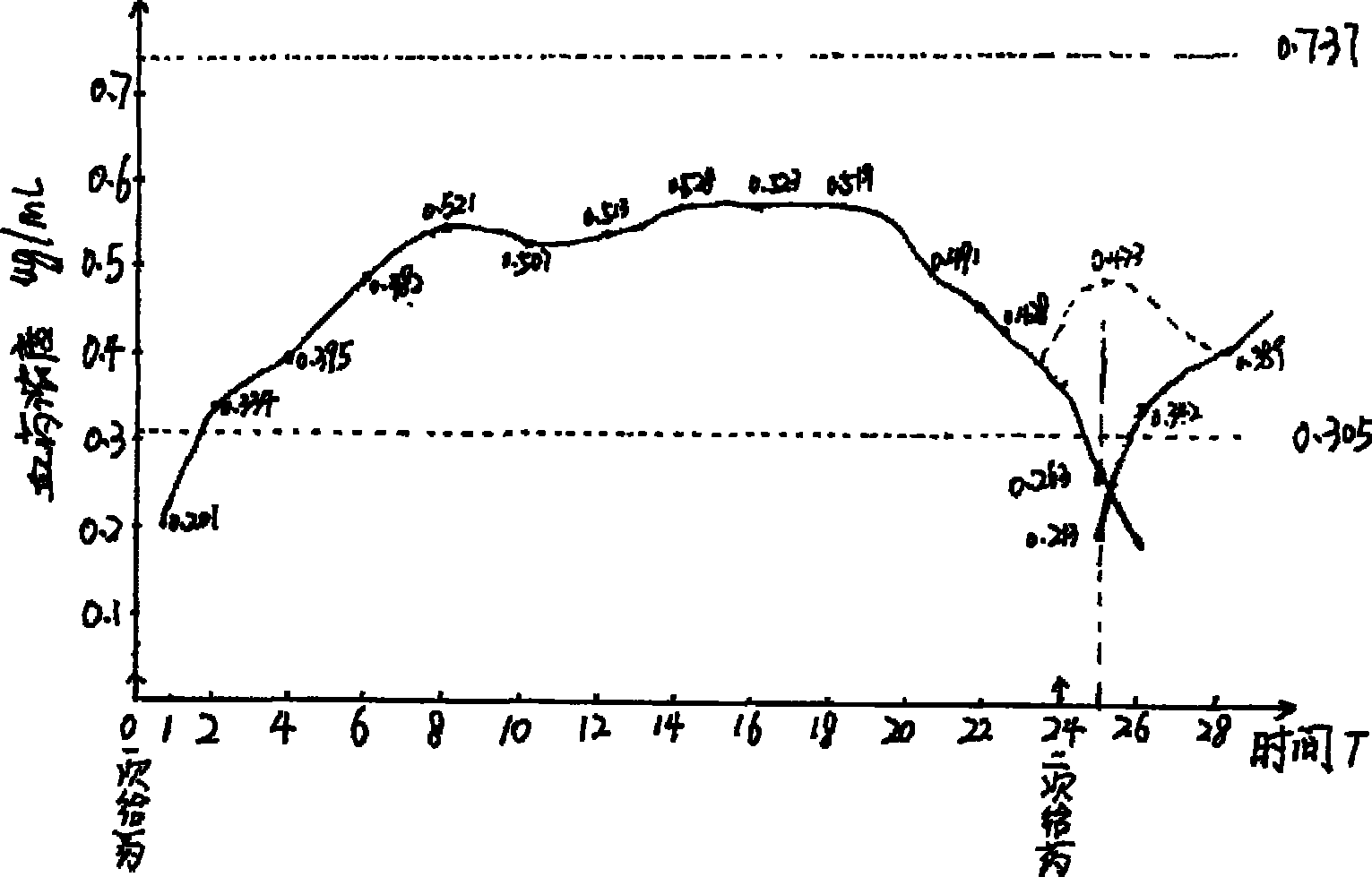
Image
Examples
Embodiment 1
[0023] 1000 tablets, the specification is 90mg.
[0024] prescription:
[0025] Tablet core Film coating
[0026] Tramadol Hydrochloride 90g Hydroxypropyl Methylcellulose 3g
[0028] Lactose 17.9g Stearic acid 0.5g
[0029] Polyvinylpyrrolidone 5.36g Ethanol 100ml
[0030] Magnesium stearate 1.78g
[0031] Glyceryl monostearate 22.5g
[0032] Proper amount of ethanol
[0033] Preparation:
[0034] a. Preparation of tablet core: Take the prescribed amount of slow-release material, add distilled water, stir in a hot water bath at 70°C to 80°C, take it out after dissolving, quickly emulsify with tramadol hydrochloride, add monostearic acid under constant stirring Glyceride until mixed evenly, adjust the pH value to 5.0-6.0 with 10% hydrochloric acid solution, add distilled water to continue stirring, then place in a cold water bath to cool, add 36% formaldehyde solution and stir for 10min-15min, adjust with 10% sodium hydroxide solution W...
Embodiment 2
[0037] 1000 tablets, the specification is 100mg.
[0038] prescription:
[0039] Tablet core Film coating
[0040] Tramadol Hydrochloride 100g Hydroxypropyl Methylcellulose 4.5g
[0041] Xanthan Gum 140g Talc 3g
[0042] Lactose 30.25g Stearic acid 0.75g
[0043] Polyvinylpyrrolidone 9.08g Ethanol 150ml
[0044] Magnesium stearate 3.03g
[0045] Glyceryl monostearate 24g
[0046] Proper amount of ethanol
[0047] The preparation method is the same as in Example 1.
Embodiment 3
[0049] 1000 tablets, the specification is 110mg.
[0050] prescription:
[0051] Tablet core Film coating
[0052] Tramadol Hydrochloride 110g Hydroxypropyl Methylcellulose 6g
[0054] Lactose 45.2g Stearic acid 1g
[0055] Polyvinylpyrrolidone 13.56g Ethanol 200ml
[0056] Magnesium stearate 4.52g
[0057] Glyceryl monostearate 25.3g
[0058] Proper amount of ethanol
[0059] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com