Soluble TNF acceptor mutant
A soluble and mutant technology, applied to cytokine/lymphokine/interferon receptors, receptors/cell surface antigens/cell surface determinants, allergic diseases, etc., can solve the problem of decreased drug efficacy and increased serious infections Incidence rate, faster drug clearance rate, etc., to reduce inhibition and affinity reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1, TNFR75 (W89L) mutant: the preparation of Fc fusion protein
[0080] (1) Preparation of TNFR75(W89L) mutant: Fc fusion protein encoding gene
[0081] Using the wild-type human soluble TNF p75 receptor: Fc fusion protein DNA fragment as a template, the mutant soluble TNF p75 receptor DNA coding sequence was obtained by SOE-PCR (Splicing by Overlapping Extension PCR) technology. First, the wild-type soluble TNFp75 receptor: Fc fusion protein coding DNA was used as a template, with primers
[0082] TNFR75p: aagcttatggctcccgtcgccgtctggg
[0083] W89LpF1: CTCAAGCACTCGGGAACcagGTTCC
[0084] Amplifies the DNA fragment of the first half of the selective soluble TNF p75 receptor containing the mutation site; with primers
[0085] Fcp: gaattcctatttacccggagacagggg
[0086] W89LpR1: GGAACctgGTTCCCGAGTGCTTGAG
[0087] Amplify selective soluble TNF p75 receptor DNA fragment including the mutation site and the second half of the Fc fragment. Finally, using the above...
Embodiment 2-8
[0098] Embodiment 2-8, other TNFR75 mutants: the preparation of Fc fusion protein
[0099] The shared primers used to prepare mutant TNFR75(W89H), TNFR75(W89Y), TNFR75(W89F), TNFR75(W89R), TNFR75(W89K), TNFR75(W89M), TNFR75(W89I):Fc fusion protein encoding gene are as follows:
[0100] TNFR75F: aagcttatggctcccgtcgccgtctggg
[0101] Fcp: gaattcctatttacccggagacagggg
[0102] The specific primers used to prepare mutant TNFR75(W89H), TNFR75(W89Y), TNFR75(W89F), TNFR75(W89R), TNFR75(W89K), TNFR75(W89M), TNFR75(W89I) TNFR75:Fc fusion protein coding gene are as follows surface:
[0103] mutant name
[0104] TNFR75(W89I)
[0105] Other operations are the same as in Example 1.
Embodiment 9
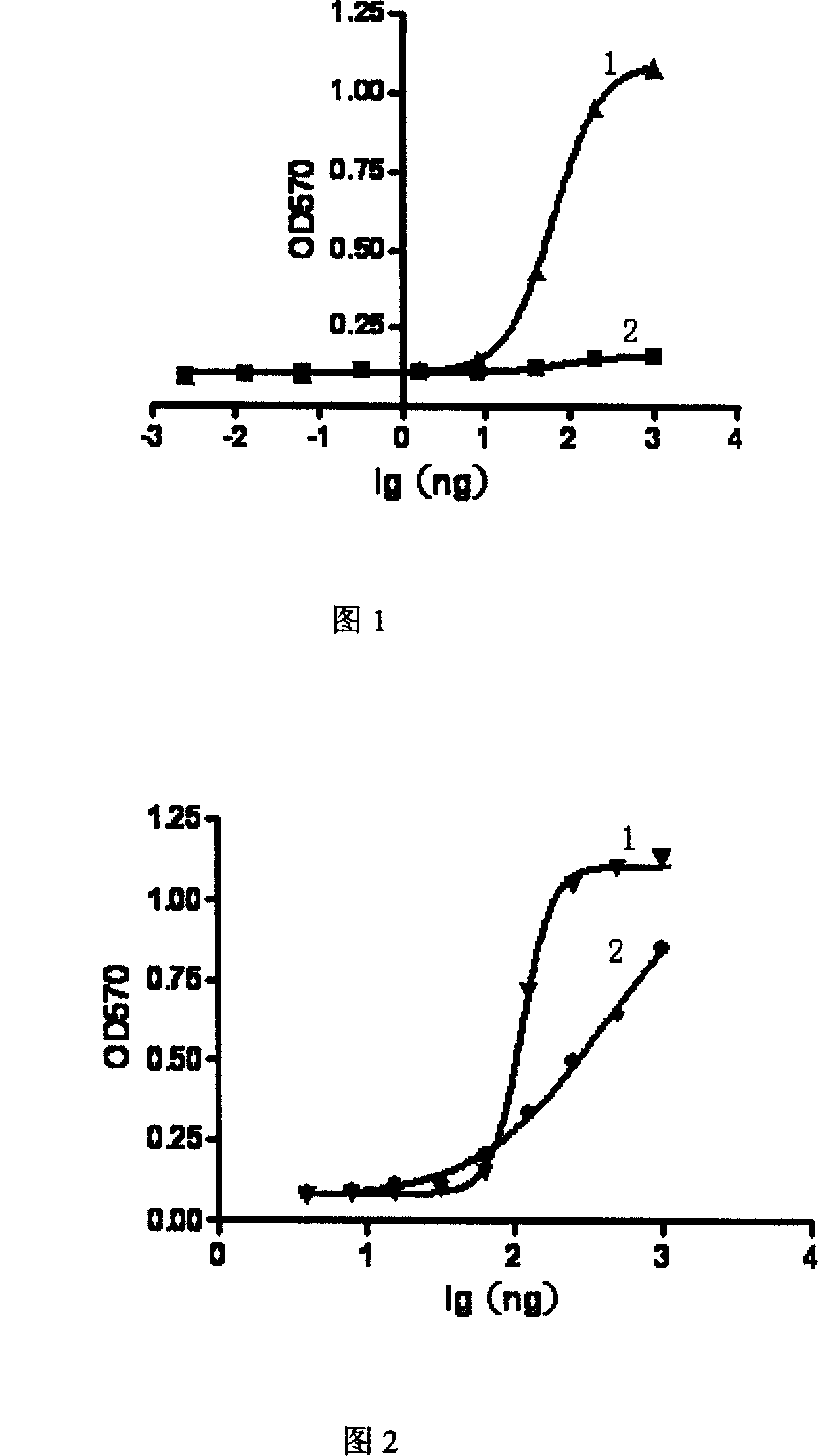
[0106] Example 9, TNFR75 mutant: Determination of Fc fusion protein and ligand binding ability
[0107] 100ul of TNF and LT28-171 were coated onto the microtiter plate at a concentration of 10ug / ml, and 100ul of gradient TNFR75(W89H) mutant: Fc fusion protein was added to each well, and repeated wells were kept at 37°C for 1 hour. Add enzyme-linked antibody goat anti-humanIgG Fc-HRP (PIERCE company): 1:20000, 100ul / well, 37°C for 1 hour. OPD color development.
[0108] TNFR75(W89L), TNFR75(W89Y), TNFR75(W89F), TNFR75(W89R), TNFR75(W89K), TNFR75(W89M), TNFR75(W89I) were operated in the same way, and the results are shown in Table 1.
[0109] Table 1. Experimental results of ligand-selective receptor binding ability
[0110] Sample serial number
[0111] TNFR2-Fc W89M
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com