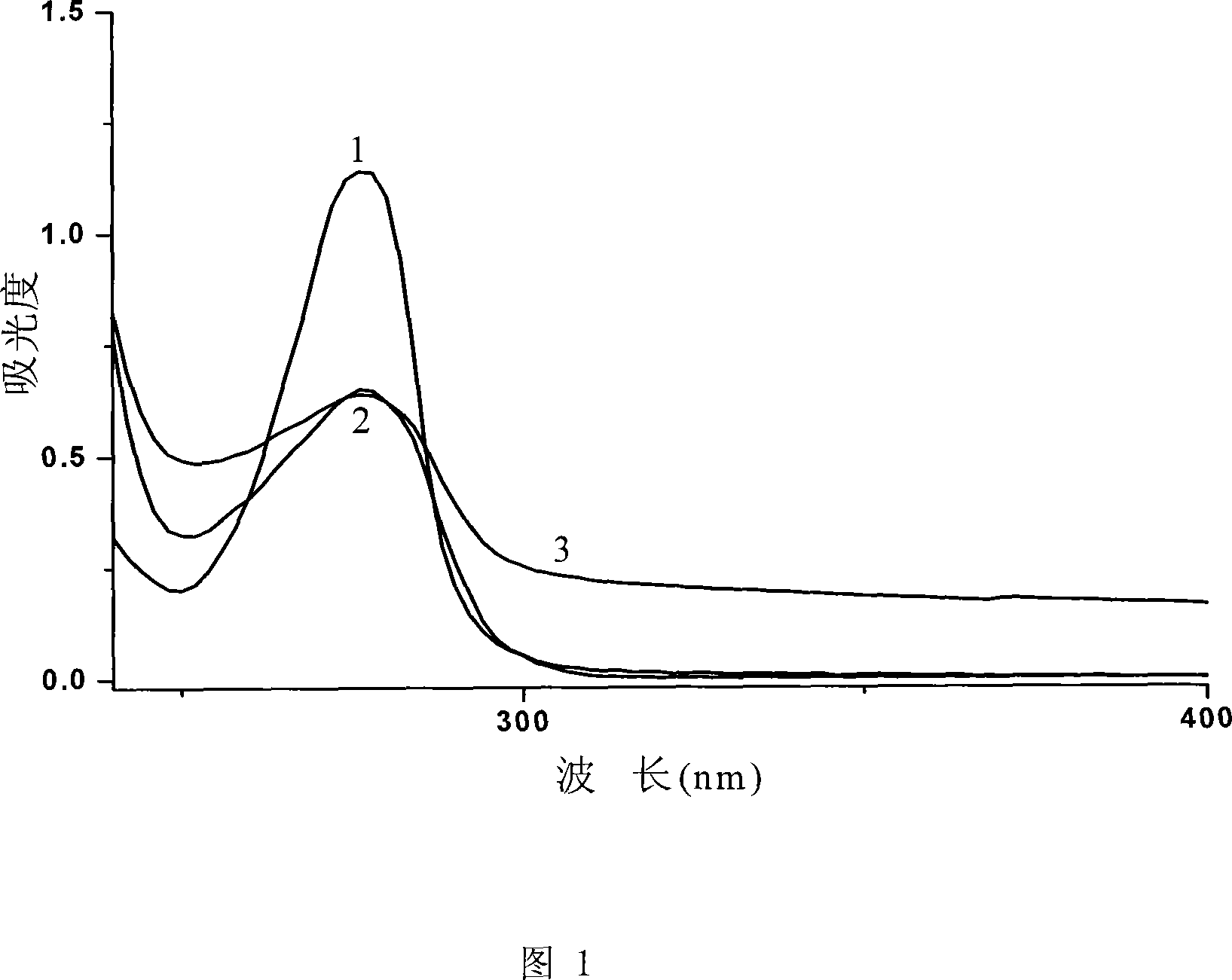
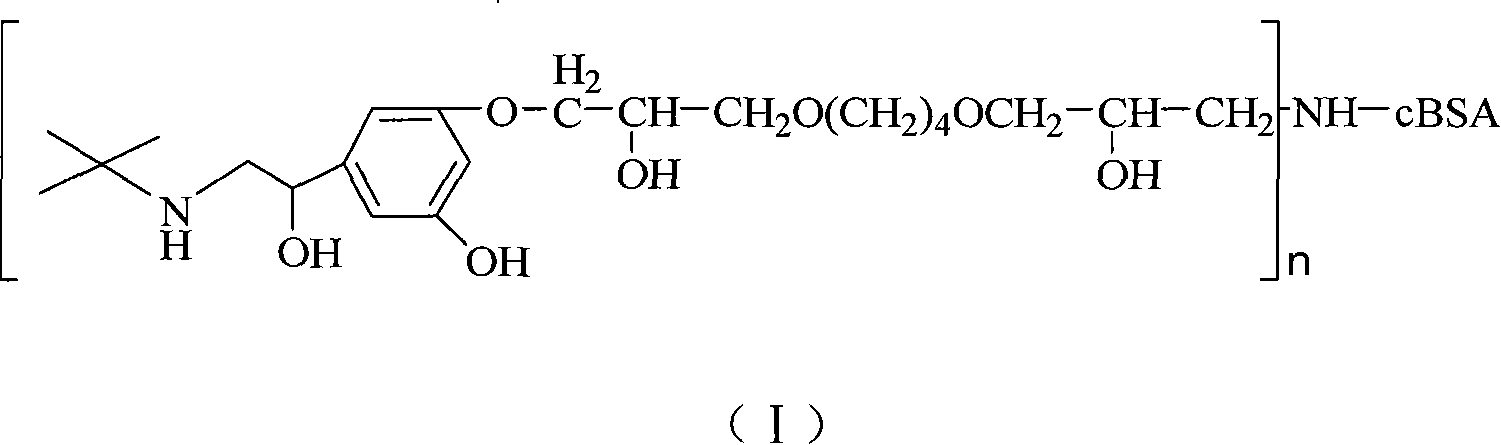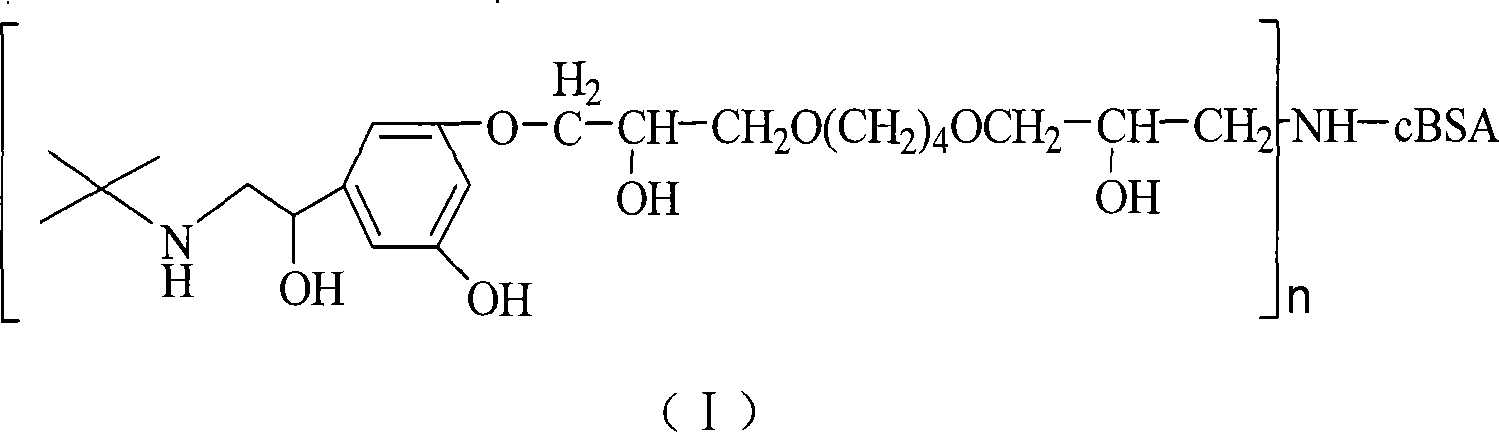Terbutaline conjugate and its preparation method and uses
A technology of terbutaline and conjugates, applied in the field of terbutaline conjugates and its preparation, to achieve the effect of saving inspection time and making up for the long time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Preparation of solution A: Weigh 50 mg cBSA (0.735 μmol) and dissolve it in 3 mL of 50 mM carbonate (pH=10.7) buffer solution, then add 13.8 μL (72.9 μmol) of 1,4-butanediol to the solution Ether (BDE), reacted at room temperature for 20 hours to obtain a colorless and clear solution A for subsequent use;
[0041](2) Preparation of solution B: Weigh 30 mg (109.4 μmol) of terbutaline sulfate and dissolve it in 1.5 mL of 50 mM carbonate (pH=10.7) buffer solution, blow solution A into nitrogen, and then dissolve terbutaline sulfate The Lin solution was added dropwise to the A solution, and reacted at room temperature for 20 hours to obtain a colorless and clear solution B.
[0042] (3) Stir the dialysis solution B for 70 to 80 hours at 0-4°C with a phosphate buffer solution with a pH of 7.40 and a concentration of 0.01M, then dialyze with distilled water for 20 to 30 hours, and change the dialysate every 6 hours;
[0043] (4) Freeze-dry the dialyzed solution to obtain...
Embodiment 2
[0046] (1) Preparation of solution A: Weigh 50 mg cBSA (0.735 μmol) and dissolve it in 3 mL of 50 mM carbonate (pH=11.1) buffer solution, then add 16.7 μL (88.2 μmol) of BDE to the solution, and react at room temperature for 22 hours , to obtain a colorless and clear solution A, for subsequent use;
[0047] (2) Preparation of solution B: Weigh 25 mg (91.2 μmol) of terbutaline sulfate and dissolve it in 1.0 mL of 50 mM carbonate (pH=11.1) buffer solution, blow solution A into nitrogen, and then dissolve terbutaline sulfate The Lin solution was added dropwise to the A solution, and reacted at room temperature for 24 hours to obtain a colorless and clear solution B.
[0048] (3) Stir the dialysis solution B for 70-80 hours at 0-4°C with a phosphate buffer solution with a pH of 7.50 and a concentration of 0.01M, and then dialyze with distilled water for 20-30 hours, changing the dialysate every 6 hours;
[0049] (4) Freeze-dry the dialyzed solution to obtain a white terbutaline c...
Embodiment 3
[0051] (1) Preparation of solution A: Weigh 60 mg cBSA (0.882 μmol) and dissolve it in 4 mL of 50 mM carbonate (pH=10.9) buffer solution, then add 25.0 μL (132.3 μmol) of BDE to the solution, and react at room temperature for 24 hours , to obtain a colorless and clear solution A, for subsequent use;
[0052] (2) Preparation of solution B: Weigh 40 mg (146 μmol) of terbutaline sulfate and dissolve it in 3 mL of 50 mM carbonate (pH=10.9) buffer solution, blow solution A into nitrogen, and then dissolve the terbutaline sulfate solution Add dropwise to solution A, and react at room temperature for 24 hours to obtain colorless and clear solution B.
[0053] (3) Stir the dialysis solution B for 70 to 80 hours at 0-4°C with a phosphate buffer solution with a pH of 7.30 and a concentration of 0.02M, and then dialyze with distilled water for 20 to 30 hours, changing the dialysate every 6 hours;
[0054] (4) Freeze-dry the dialyzed solution to obtain a white terbutaline conjugate, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com