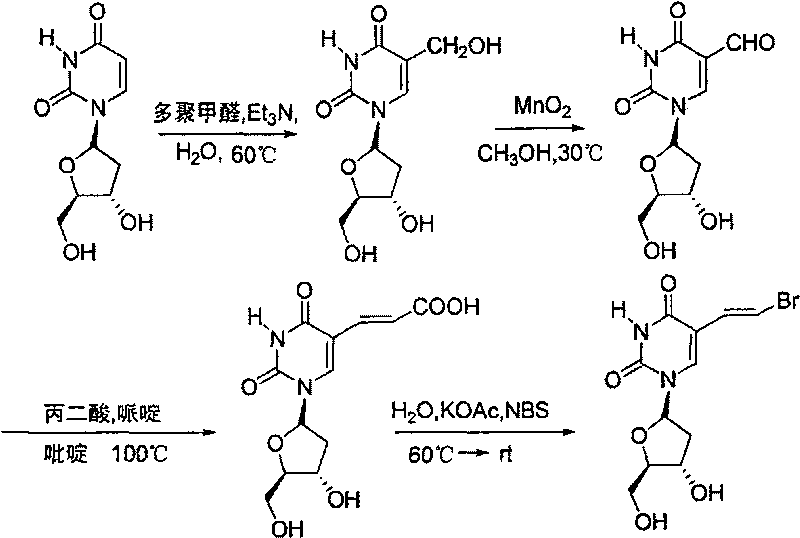
Preparation process of brivudine
A technology of bromofuridine and deoxyuracil, applied in the field of medicine, can solve problems such as isomerization of glycoside and bromopyrimidine, and achieve the effects of high reaction yield, reduction of production cost, and improvement of total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Preparation of 5-hydroxymethyl-2'-deoxyuracil (I)
[0029] 2'-deoxyuracil (5.25g, 23.1mmol) and paraformaldehyde (3.11g, 103.5mmol) were dissolved in 0.5M triethylamine aqueous solution (80ml), heated up to 60°C, reacted for four days, and added Paraformaldehyde (4.49g, 149.5mmol; 3.21g, 105.8mmol) was added once, triethylamine (1ml) and water (10ml) were added once in the middle, and finally concentrated and recrystallized in methanol to obtain 4.11g of white solid, namely: 5-Hydroxymethyl-2'-deoxyuracil (I), yield 70%.
[0030] Its characteristic parameters are: 1 H NMR (400MHz, DMSO-d6) δ: 11.34(s, 1H, N-H), 7.71(s, 1H, 6-H), 6.17(t, J=6.8Hz, 1H, 1'-H), 5.26( s, 1H, 3'-OH), 4.97 (s, 2H, 5'-OH and CH 2 O-H), 4.20(q, J=3.2Hz, 1H, 4'-H), 4.10(s, 2H, 5-H), 3.75(q, J=3.2Hz, 1H, 3'-H), 3.57- 3.47(m, 2H, 5'-H), 2.09-1.99(m, 2H, 2'-H)).
[0031] (2) Preparation of 5-formyl-2'-deoxyuracil (II) from compound (I)
[0032] (1) (0.70g, 2.7mmol) and newly prepared mangan...
Embodiment 2
[0041] (1) Preparation of 5-hydroxymethyl-2'-deoxyuracil (I)
[0042] 2'-Deoxyuracil (15.25g, 69.3mmol) and paraformaldehyde (9.96g, 311.9mmol) were dissolved in 0.5M triethylamine aqueous solution (240ml), heated to 60°C, reacted for four days, then every other day Add paraformaldehyde (14.38g, 450.5mmol; 10.18g, 318.8mmol) once, add triethylamine (3ml) and water (30ml) once in the middle, and finally concentrate and recrystallize with methanol to obtain 10.01g of 5-hydroxymethyl Base-2'-deoxyuracil (I), the yield was 56%.
[0043] (2) Preparation of 5-formyl-2'-deoxyuracil (II) from compound (I)
[0044] (I) (1.01g, 3.9mmol) and manganese dioxide (7.78g, 89.5mmol) in 20ml DMF (N, N-dimethylformamide), after reacting at 10 ℃ for 4 days, filter with diatomaceous earth , the resulting filtrate was concentrated and recrystallized in methanol to obtain 0.60 g of 5-formyl-2'-deoxyuracil (II), with a yield of 61%.
[0045] (3) Preparation of (E)-5-(2-carboxyvinyl)-2'-deoxyuracil...
Embodiment 3
[0050] (1) Preparation of 5-hydroxymethyl-2'-deoxyuracil (I)
[0051] The synthesis method refers to (1) in Example 1.
[0052] (2) Preparation of 5-formyl-2'-deoxyuracil (II) from compound (I)
[0053] (I) (0.20g, 0.8mmol) and manganese dioxide (1.54g, 8mmol) (10eq.) in 5ml DMF (N,N-dimethylformamide), after reacting at 10°C for 9 hours, use silicon Manganese dioxide was removed by algal earth suction filtration, concentrated, and passed through a column with ethyl acetate to obtain 0.08 g of 5-formyl-2'-deoxyuracil (II), with a yield of 42%.
[0054] (3) Preparation of (E)-5-(2-carboxyvinyl)-2'-deoxyuracil (III) from compound (II)
[0055] The synthesis method refers to (3) in Example 1.
[0056] (4) Preparation of (E)-5-(2-bromovinyl)-2'-deoxyuracil (IV) from compound
[0057] The synthesis method refers to (4) in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com