Medicinal composition and its preparation method and quality control method
A quality control method and composition technology, applied in the pharmaceutical composition of ankylosing spondylitis and preparation thereof, in the field of treating rheumatoid arthritis, and can solve the problem that rheumatoid arthritis and ankylosing spondylitis are not effectively controlled, etc. problem, to achieve the effect of accurate dose, stable quality and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0031] Experimental example 1 Pharmacodynamic experiment of tablet of the present invention
[0032] Wistar rats were randomly divided into groups according to body weight and sex, and administered by intragastric administration.
[0033] ①Treatment of experimental arthritis
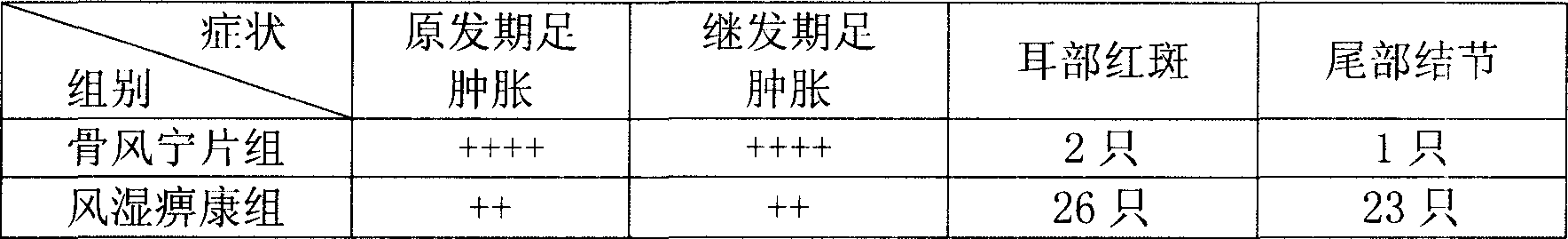
[0034]
[0035] Note: + is the improvement degree of treatment
[0036]Through the rat adjuvant arthritis experiment, Gufengning Tablets had a significant inhibitory effect on the primary and secondary stages of adjuvant arthritis in rats. Compared with the control group, Fengshibikang Capsules were significantly relieved. During the whole experiment, there was no significant reduction in food intake and changes in appearance and morphology of the animals.
[0037] Group, dose (mg / kg)
[0038] Through the experiment on the delayed type hypersensitivity reaction of mouse auricle skin caused by DNCB, Gufengning tablet has obvious inhibitory effect on the delayed type hypersensitivity reacti...
experiment example 3
[0047] Experimental Example 3 Research on the Extraction Process of the Composition of the Present Invention
[0048] 1. Screening of process conditions
[0049] (1) Source, identification and pre-treatment of medicinal materials
[0050] Among the eleven herbs in the prescription, except Kunming Shanhaitang, Yunweiling, Yexiahua, and Zidanshen, which are respectively recorded in "Guangxi Traditional Chinese Medicinal Materials Standard" and "Yunnan Provincial Drug Standard", the remaining seven herbs are all recorded in "China Pharmacopoeia 2005 edition one. The impurities and non-medicinal parts of each medicinal material are picked out, and the dust is screened out, and they are used as medicine after passing the inspection.
[0051] (2) Inspection of the crushed part
[0052] the batch
Total amount of medicinal materials (g)
Powder weight (g)
Powder yield%
1
2
3
5000
5000
5000
4259.1
4413.0
4378.4
85....
experiment example 4
[0063] Experimental example 4 Concentration and drying process research
[0064] 1. Research on concentration process
[0065] The degree of concentration is measured by relative density. If it is too low, it will prolong the drying time. If it is too high, it will stick to the pan or even gelatinize. The composition is different and the properties of the extract are different, so the concentrated relative density will also be different. Therefore, the properties of the concentrated paste directly affect the amount of paste received, the effect of mixing and the difficulty of drying. Through the pilot test, it is determined that the medicinal solution is concentrated to a clear ointment with a relative density of 1.34-1.36 at 50°C, which is easy to collect, mix and dry.
[0066] Weigh each medicinal material according to the proportion of the prescription, and use 70% ethanol (6 times, 4 times, 4 times the amount) for each part of Kunming Begonia to reflux extract three times...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com