Silicide compositions containing alkali metals and methods of making the same
A technology of alkali metal silicide and composition, which is applied in the production of metal silicide, hydrogen, phosphate material processing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0039] In each of the following examples, an easily evacuable Erlenmeyer flask was used to prepare samples of alkali metal silicide materials. Insert the silicon into the flask and connect it to the vacuum line with UltraTorr fittings and Kontes vacuum valve. The flask was then heated with a flame under vacuum and inserted into a glove box filled with helium, where sodium blocks had been added. After taking it out of the box, the flask was evacuated again and the sodium lumps were melted. When the pressure stabilizes at about 3×10 -5 After holding, seal the tube from above the flask, and heat the flask and its contents in an oven at the desired reaction temperature overnight or longer. When the reaction is complete, the flask is inserted into the glove box again, the soldered pins are broken, and the contents are taken out and stored for further study.
[0040] Example 1-Initial Test
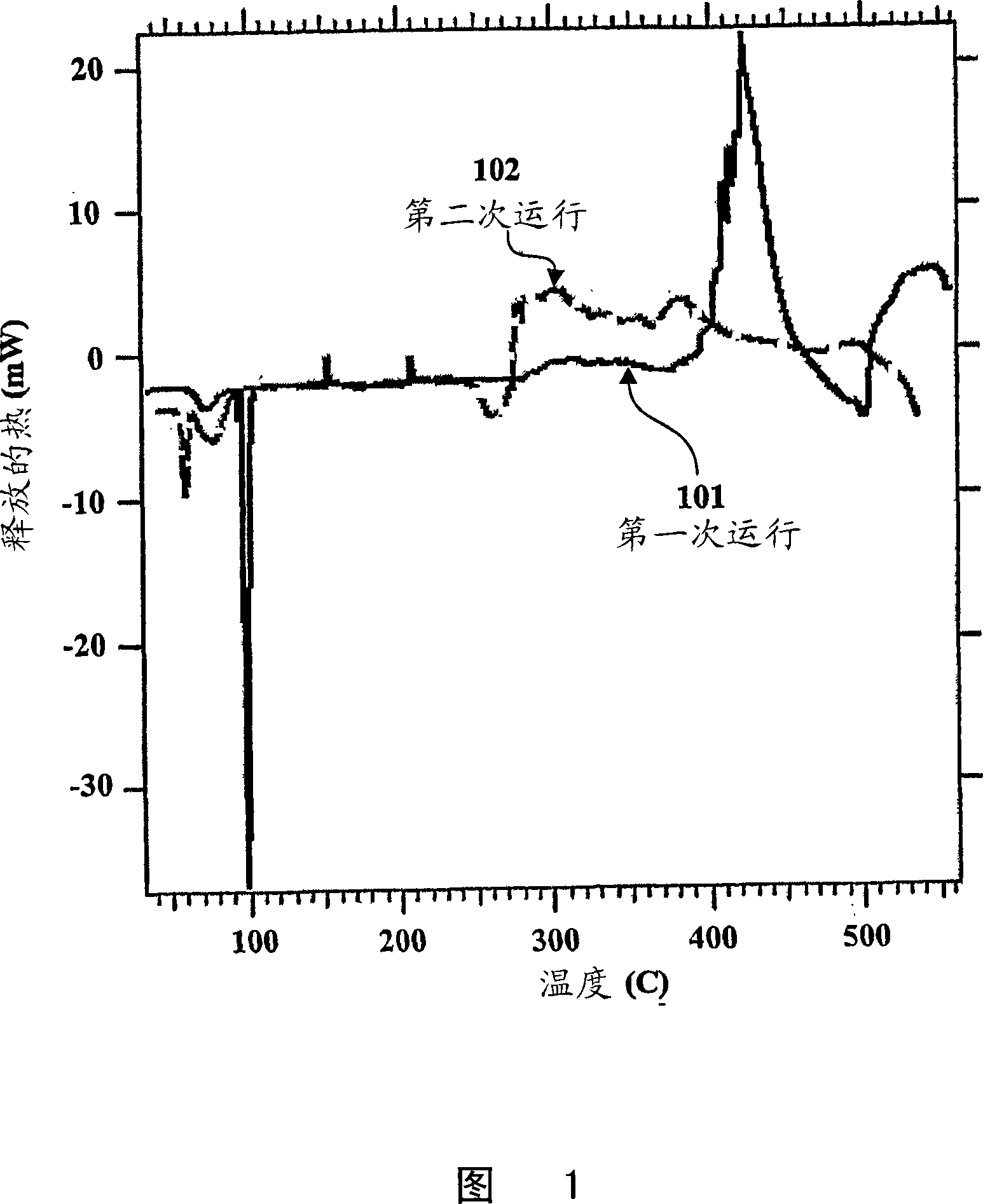
[0041] Although it is known that Na reacts with borosilicate glass at temperatures exceeding 300...
Embodiment 2
[0046] Example 2-Stability in air
[0047] During the process, the Na-Si material of the present invention immediately reacts with water to generate hydrogen gas and release heat. However, the material is completely unreactive to dry oxygen for at least one day. Unless the relative humidity is high, the powder can be weighed in the air or transferred from one container to another. The sample is exposed to the laboratory air in the aluminum weighing pan, but reacts slowly with moisture. After 2 hours, adding a small amount of liquid water, the black block immediately precipitated hydrogen. It may be that the method of preparing the composition of the invention described herein produces an alkali metal silicide protected by a surface layer of silicon dioxide or some other composition. In any case, the resulting material is easy to handle in air, which creates the ability to generate hydrogen when needed.
[0048] These results indicate that it can directly produce silicides that may...
Embodiment 3-500
[0049] Example 3-Reaction between sodium and silicon powder at 500°C
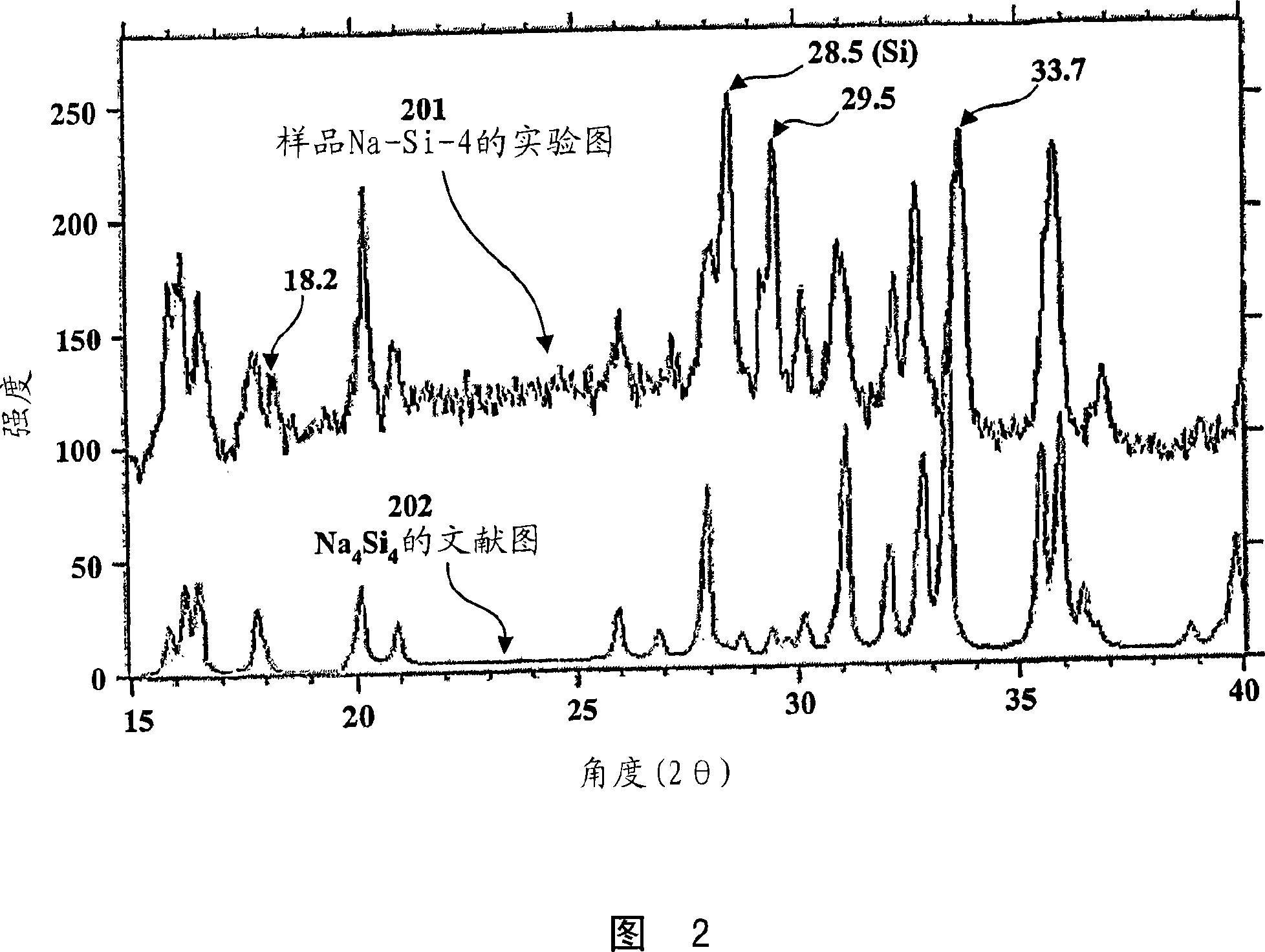
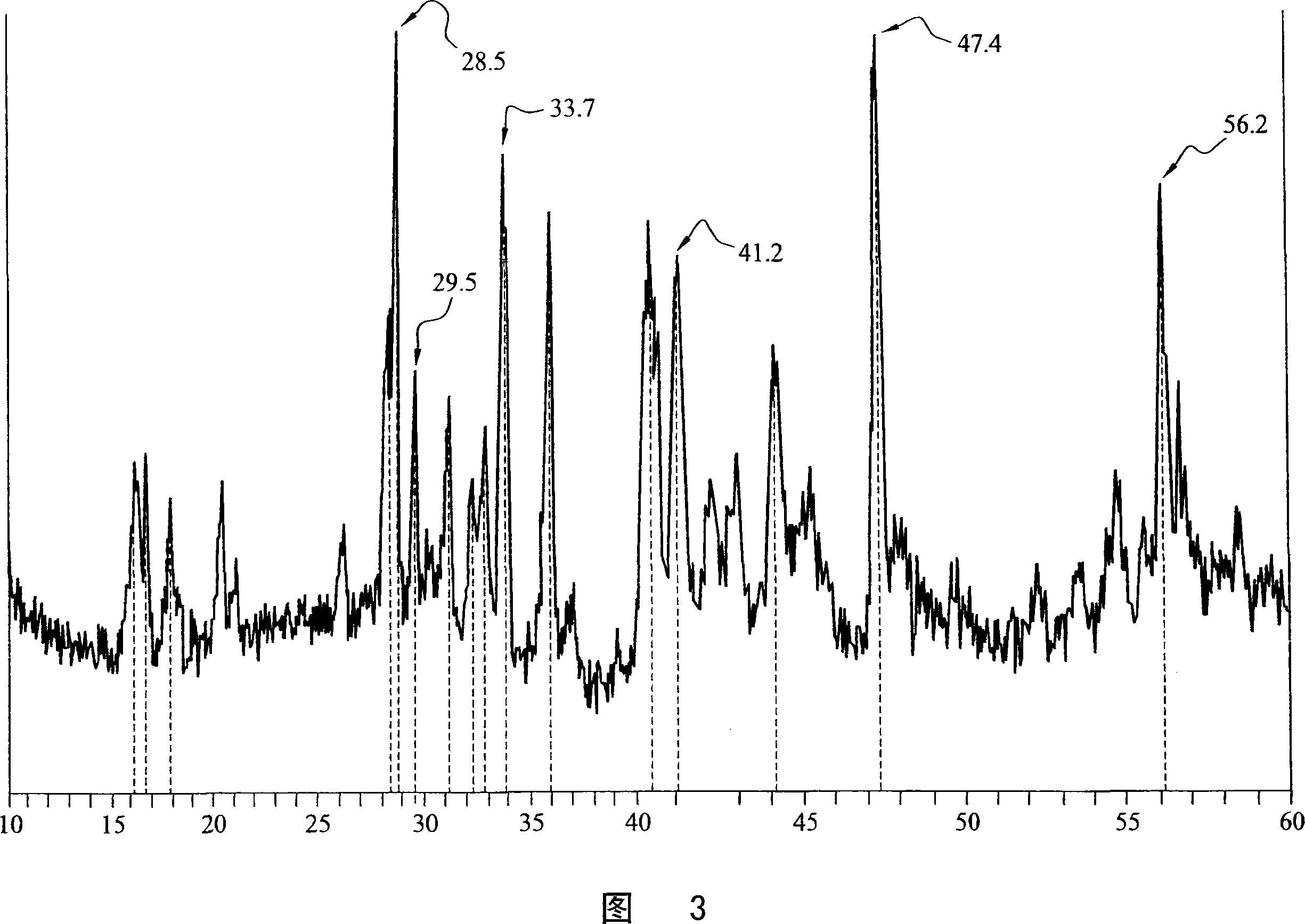
[0050] The sample Na-Si-1 was prepared by introducing 0.56 g of powdered crystalline silicon (Alfa Aesar, 325 mesh) into an easily evacuable Erlenmeyer flask. After degassing with a gas-oxygen torch (~300°C) under vacuum, the flask was placed in a helium-filled glove box, and 0.46 g of sodium metal was added. After evacuating and melting the sodium, the bottle neck was sealed under vacuum, and the flask and contents were heated in a furnace for 2 hours at 300°C, 22 hours at 400°C, and 48 hours at 500°C. The Pyrex flask turned dark brown-red in the process, and the product consisted of powder and a small amount of lumps. In the glove box, 0.66 g of product was recovered. When water was added, the 24.5 mg sample produced 0.517 millimoles (mmol) of hydrogen. This corresponds to 0.0423kg H 2 / kg sample. If hydrogen is produced only by sodium silicide, the quantity corresponds to 43% Na 4 Si 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com