Monoamine oxidase activity fluorescent detecting method
A technology of monoamine oxidase activity and monoamine oxidase, which is applied in the field of fluorescence detection of monoamine oxidase activity, can solve problems such as complex preparation, and achieve the effects of simple equipment, simple operation process and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
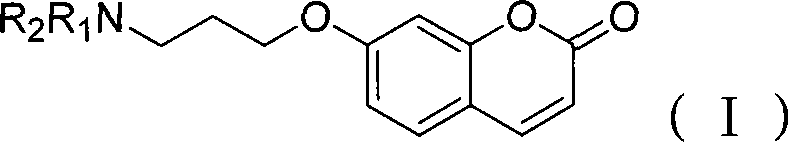
[0026] Taking monoamine oxidase extracted from rat liver as an example, the prepared 7-(3-amino-propoxy)-coumarin was used as a fluorescent probe to detect monoamine oxidase activity:
[0027] Dissolve the test sample of monoamine oxidase extracted from rat liver in a 1ml EP tube, take 100uL and put it into 900uL 50mM pH8.4 boric acid buffer, place it in a constant temperature water bath shaker, preheat at 35°C for 5min, add 5μl 7-(3-amino-propoxy)-coumarin at a concentration of 10 mM was reacted with 2 μl of 1 g / mL BSA reaction solution. After the reaction, it was detected in a 96-well sieve plate with a fully functional fluorescence spectrophotometer.
[0028] Experiments have proved that as long as there is a small amount of monoamine oxidase (5-10mmol / L), the fluorescent probe can be efficiently hydrolyzed. The fluorescence intensity measured by the full-featured microplate detection system increases from 1000 to 20000. The change range is large and intuitive. The change ...
Embodiment 2
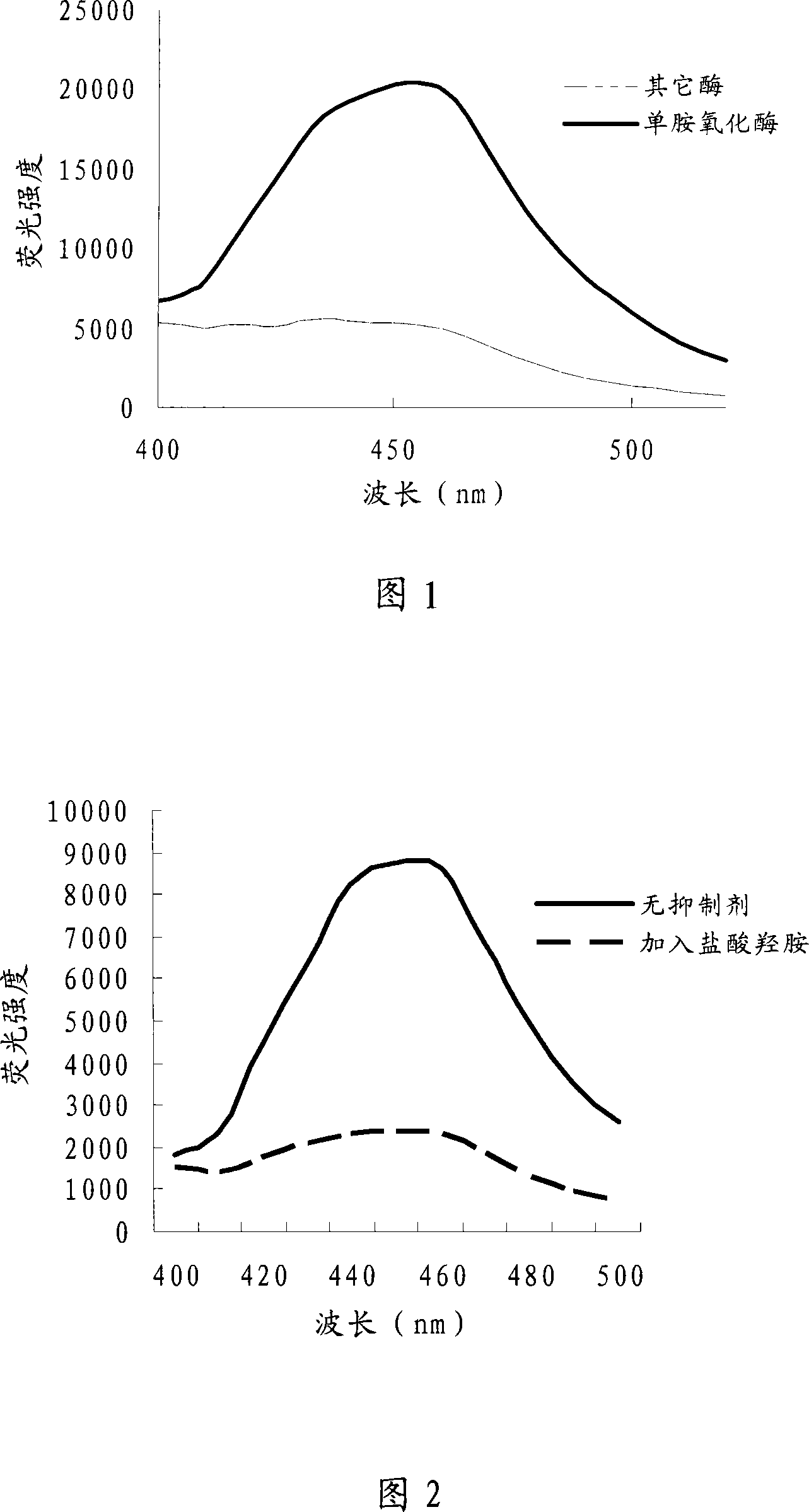
[0030] Using the same method as in Example 1, the following enzyme preparations are used to act on the substrate: lipase (EC3.1.1.3 Type VII from Candida rugosa, and hog pancreas Sinopharm Chemical Reagent Co., Ltd.), phosphatase (Phosphatase, Alkalinebovine ), multiple proteases (Protease from Aspergillus oryzae, Protease from Aspergillus sojae, Protease from bovine pancreas) and a compound enzyme (BS-031 Jiangmen Lianjiang District Biochemical Factory), the results showed that only monoamine oxidase showed strong fluorescence , the results are shown in Figure 1, only monoamine oxidase can convert the substrate, which also shows that the monoamine oxidase we extracted is responsible for the hydrolysis, while other enzymes have no effect on the substrate.
Embodiment 3
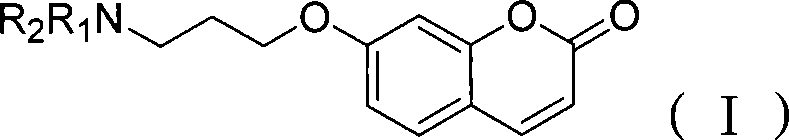
[0032] Take 100 μl of boric acid buffer solution with pH 8.4, add 15 μl of monoamine oxidase extract solution, 2 μl of 0.1 mM monoamine oxidase inhibitor—hydroxylamine hydrochloride solution, place in a constant temperature water bath shaker at 35°C, and after 30 minutes, add according to the amount in Example 1 7-(3-Amino-propoxy)-coumarin and BSA solution react, after the reaction, use a full-featured fluorescence spectrophotometer to detect in a 96-well sieve plate in a full-featured fluorescence spectrophotometer, and detect fluorescence in real time strength.
[0033] Experiments have proved that after the activity of monoamine oxidase is inhibited by hydroxylamine hydrochloride, no activity can be detected with our prepared probe, and the results are shown in Figure 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com