Method for separating and purifying ACE inhibition peptide from rice draff and active peptide obtained therefor
A technology of inhibiting activity and rice grains, applied in the field of bioengineering and protein chemistry, to achieve the effect of simple method, excellent product sensory and high protein content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Sephadex G-15 was used to separate and prepare the hydrolysate of rice grains to obtain the separated components:
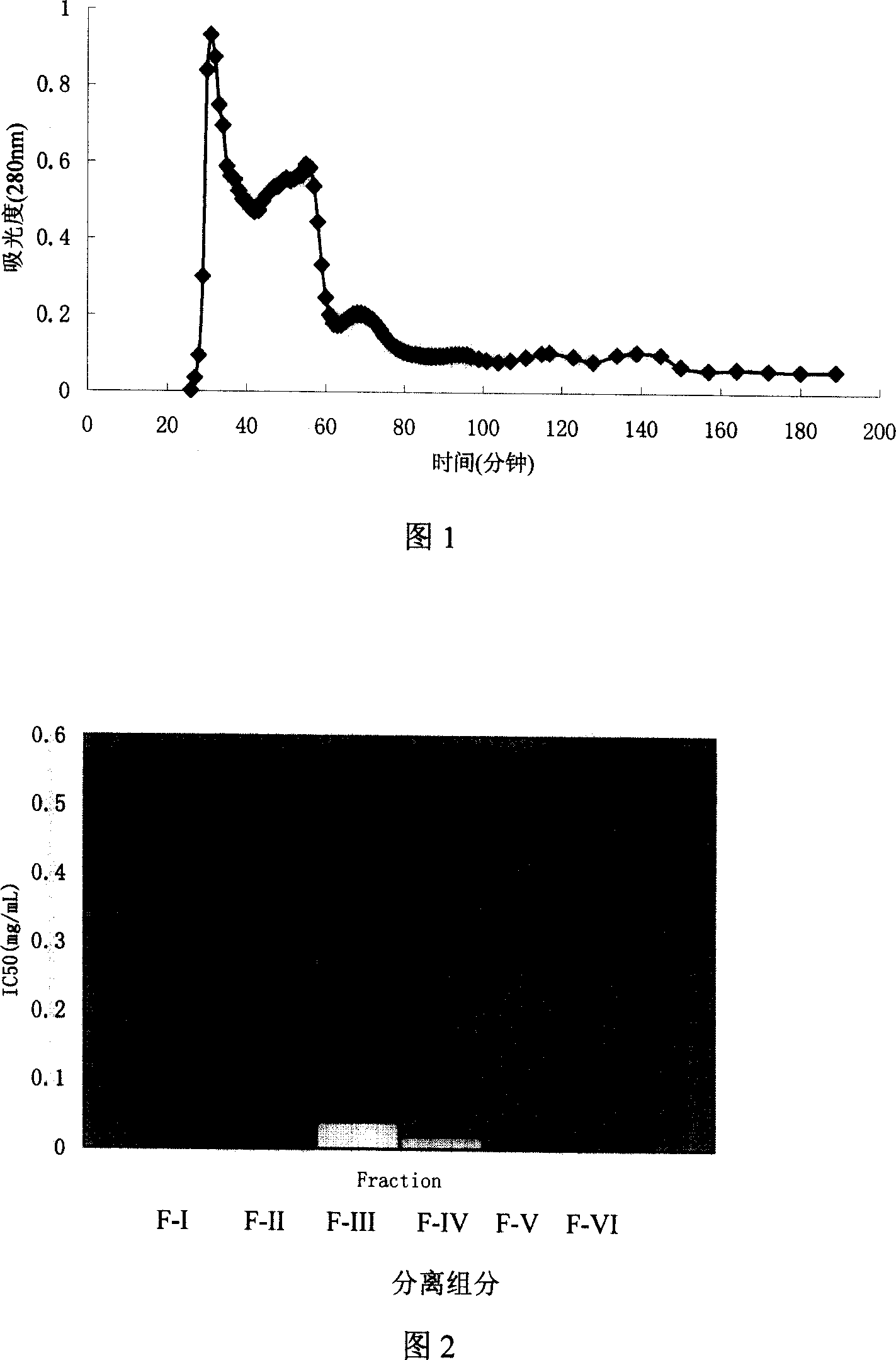
[0036] In the separation process, the present invention uses a 160cm (length)×30mm chromatography column, the detection wavelength is 280nm, and the eluent is distilled water. The eluent flow rate is 0.5ml / min, and the sample volume is 25-30ml, according to the wavelength value OD in the nucleic acid protein detection instrument 280 Change the collection of samples. According to the above given operating conditions, short peptides with ACE inhibitory activity can be collected.
[0037]As can be seen from Figure 1, the proteolysate of rice grains is separated into six distinct polypeptide components after separation by Sephadex G-15, which are named FI, F-II, F-III, and F respectively. -IV, FV, F-VI. The peak areas of components FI, F-II, F-III, F-IV, FV, and F-VI separated by Sephadex G-15 are 36.08%, 45.93%, 7.98%, and 2.13%, respectively. , 3.03%,...
Embodiment 2
[0043] Example 2: Study and comparison of the half inhibitory concentration (IC50) of the ACE inhibitory activity of the separated components of Sephadex G-15 from rice grains proteolysis:
[0044] Raw materials: The method described in Example 1 was used to obtain the gel separation components of the rice grains proteolysate as the research raw materials.
[0045] In order to accurately describe the ACE inhibitory activity of components F-I, F-II, F-III, F-IV, F-V, F-VI, the half-inhibitory concentration (IC 50 ). The measurement results are shown in Figure 2. It can be seen from Figure 2 that the half-inhibitory concentration (IC) of the ACE inhibitory activity of the separated components F-I, F-II, F-III, F-IV, F-V, and F-VI 50 ) Are 0.502g / mL, 0.171mg / mL, 0.041mg / mL, 0.019mg / mL, 0.011mg / mL, 0.017mg / mL, respectively. These data indicate that F-III, F-IV, F-V, and F-VI have higher ACE inhibitory activity; among them, the component with the highest ACE inhibitory activity is F-V,...
Embodiment 3
[0049] Example 3: Chromatographic separation of F-V components separated by Sephadex G-15 from rice grains proteolysate and preparation of reversed-phase high performance liquid chromatography:
[0050] In order to further ascertain the component F-V with high angiotensin-converting enzyme (ACE) inhibitory activity, reversed-phase high performance liquid chromatography was used to separate and analyze F-V, as follows:
[0051] In this implementation, the Sephadex G-15 separation component F-V of the proteolytic product is vacuum freeze-dried to form a freeze-dried powder. The process conditions of freeze-drying are: temperature -45°C and vacuum degree 20Pa.
[0052] Then dissolve the above lyophilized powder in distilled water to make a solution with a concentration of 5mg lyophilized powder / mL, and use C 18 Reversed-phase high performance liquid chromatography was separated and analyzed. The chromatographic conditions are as follows:
[0053] Chromatographic column: C18P / N84176 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com