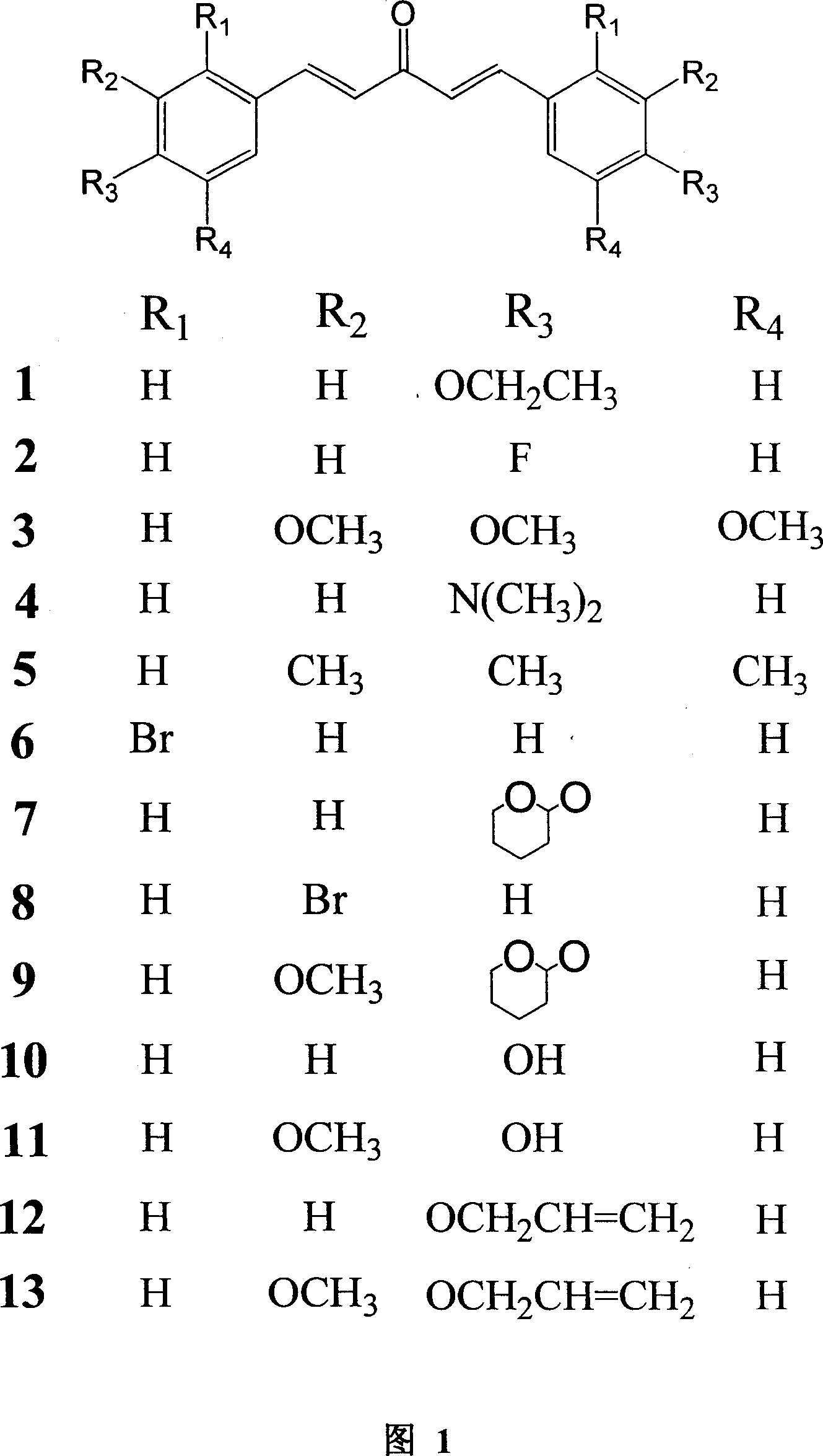
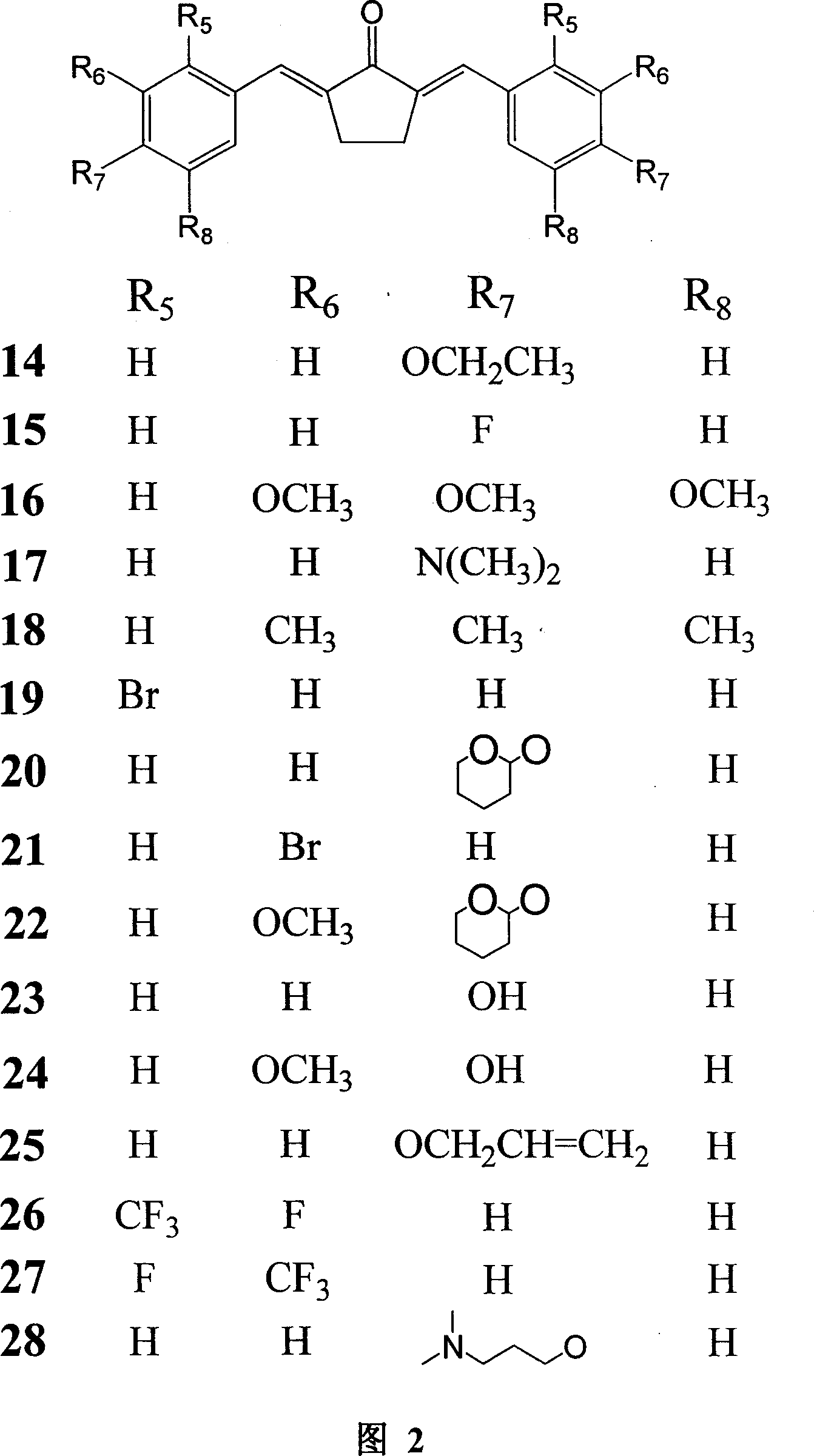
Analog of mono carbonyl structure of curcumin, and usage
A hydroxyl and halogen technology, applied in the field of structural analogs of curcumin, can solve problems such as weak stability of curcumin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0080] In the following non-limiting examples, the invention is illustrated in more detail.
[0081] A.Materials and methods
[0082] Preparation of compounds 1, 2, 3, 4, 5, 6, and 8: at room temperature, dissolve 0.01 mol of the corresponding benzene ring substitute in 10 ml of absolute ethanol, slowly add 0.005 mol of dried acetone dropwise, and stir for 10 minutes ; Slowly add 18% methanol solution containing 0.005mol sodium methoxide dropwise, stir and react for 3 to 10 hours, and a yellow precipitate is formed. After the reaction, 50ml of water was added, filtered, the filter residue was washed successively with 50ml of water and 50ml of ethanol, and vacuum-dried at 30°C. The obtained crude product was purified by silica gel column chromatography (methanol:chloroform 1:13).
[0083] Compound 1, yellow powder (56.1% yield), mp122-124°C; 1 HNMR (CDCl 3 , 400MHz) δ (ppm) 1.39 (6H, t, CH 3 ×2), 4.10 (4H, q, O-CH 2×2), 6.99(4H, m, J=7.2, Ar-H), 7.14(2H, d, J=16Hz, CH-C=O×...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com