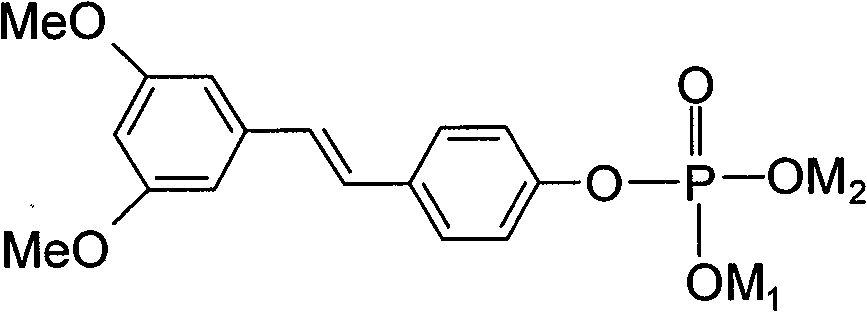
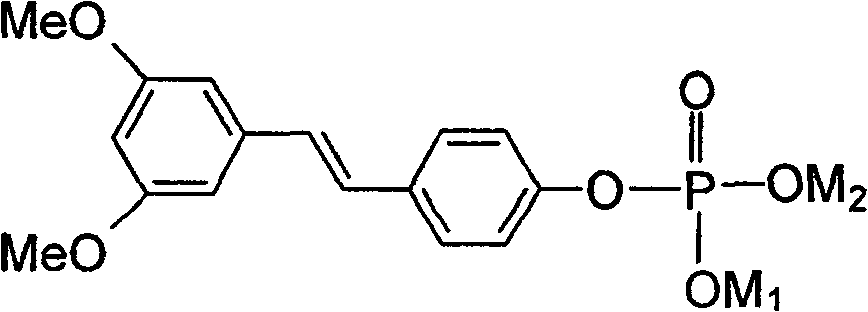
(E)3,5-di-methoxy-diphenylene-4'-0-phosphates (salt) and its preparing method, medicine composition and use
A technology of dimethoxystilbene and hydroxystilbene is applied in the field of stilbene derivatives, which can solve the problems of low biocompatibility and bioavailability, unfavorable vascular administration, low water solubility and the like, Achieving the effect of good biocompatibility, simple post-processing procedures and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of (E)-3,5-dimethoxystilbene-4'-O-phosphate disodium salt:
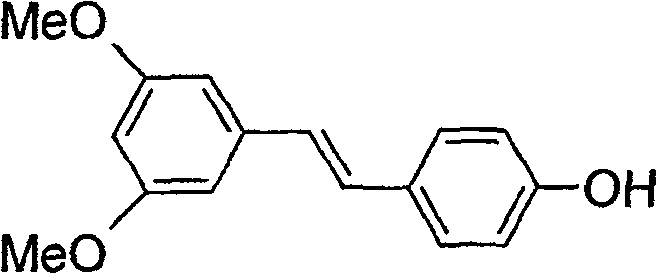
[0044] (E)-3,5-dimethoxy4'-hydroxystilbene 1.3g (0.005mol) was dissolved in 6mL CH 2 Cl 2 , then add POCl 3 2.6mL (0.028mol) was stirred well. Slowly add dropwise with 6mLCH 2 Cl 2 The dissolved 3.3 mL (0.024 mol) triethylamine was stirred for 6 hours at 25°C. After the reaction was completed, it was washed three times with an appropriate amount of water. The organic layer was dried over anhydrous magnesium sulfate and concentrated to obtain a yellow oily liquid, that is, the phosphorylated intermediate. 50 mL (0.015 mol) of NaOH solution with a concentration of 0.3 mol / L was added to the above phosphorylated intermediate concentrated solution, heated to 70-80° C. in an oil bath, and vigorously stirred to react for 8 hours. The insoluble matter in the reaction solution was removed by filtration, concentrated to dryness, and then recrystallized from a mixed solution of water and acetone to ob...
Embodiment 2
[0049] Preparation of (E)-3,5-dimethoxystilbene-4'-O-phosphate disodium salt:
[0050] (E)-3,5-dimethoxy-4'-hydroxystilbene compound 12.8g (0.05mol) was dissolved in 80mL CH 2 Cl 2 , then add POBr 3 20.5mL (0.2mol) was stirred evenly, and 16.0mL (0.2mol) pyridine and 40mL CH were slowly added dropwise 2 Cl 2 The solution was stirred at 25 °C for 4 hours. After the reaction was completed, it was washed three times with an appropriate amount of water. The organic layer was dried over anhydrous sodium sulfate, and concentrated to obtain a yellow oily liquid, which is a phosphorylated intermediate. 200 mL (0.20 mol) of 1 mol / L NaOH solution was added to the above phosphorylated intermediate concentrated solution, heated to 80° C. in an oil bath, and the reaction was stirred for 10 hours. The insolubles in the reaction solution were removed by filtration, concentrated to dryness, and then recrystallized with a mixed solution of ketone and acetone to obtain 17.0 g of a white s...
Embodiment 3
[0052] Preparation of (E)-3,5-dimethoxystilbene 4'-O-phosphate dipotassium salt:
[0053] (E)-3,5-dimethoxy-4'-hydroxystilbene compound 1.3g (0.005mol) was dissolved in 6mL CH 2 Cl 2 , then add POCl 3 2.6mL (0.028mol) was stirred well. Slowly add 1.4mL (0.02mol) trimethylamine and 6mL CH 2 Cl 2 The solution was stirred at room temperature for 8 hours. After the reaction was completed, it was washed three times with an appropriate amount of water. The organic layer was dried over anhydrous magnesium sulfate, and concentrated to obtain a yellow oily liquid, that is, the phosphorylated intermediate. 30 mL (0.015 mol) of 0.5 mol / L KOH solution was added to the above phosphorylated intermediate concentrated solution, heated to 50° C. in a water bath, and vigorously stirred to react for 6 hours. The insolubles in the reaction solution were removed by filtration, concentrated to dryness, and then recrystallized from a mixed solution of water and acetone to obtain 1.5 g of a w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com