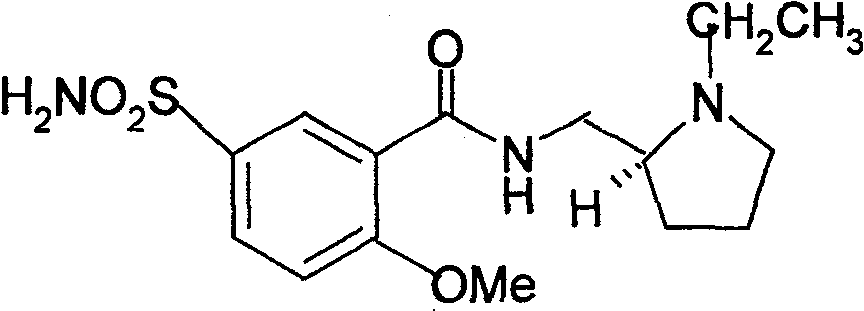
Levogyration sulpiride injection preparation method
A technology of levosulpiride and injections, which is applied in the fields of medical formula, digestive system, inorganic non-active ingredients, etc., can solve the problems of levosulpiride being insoluble in water and difficult to prepare injections, etc., to expand the scope of medicine, improve water solubility, The effect of increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
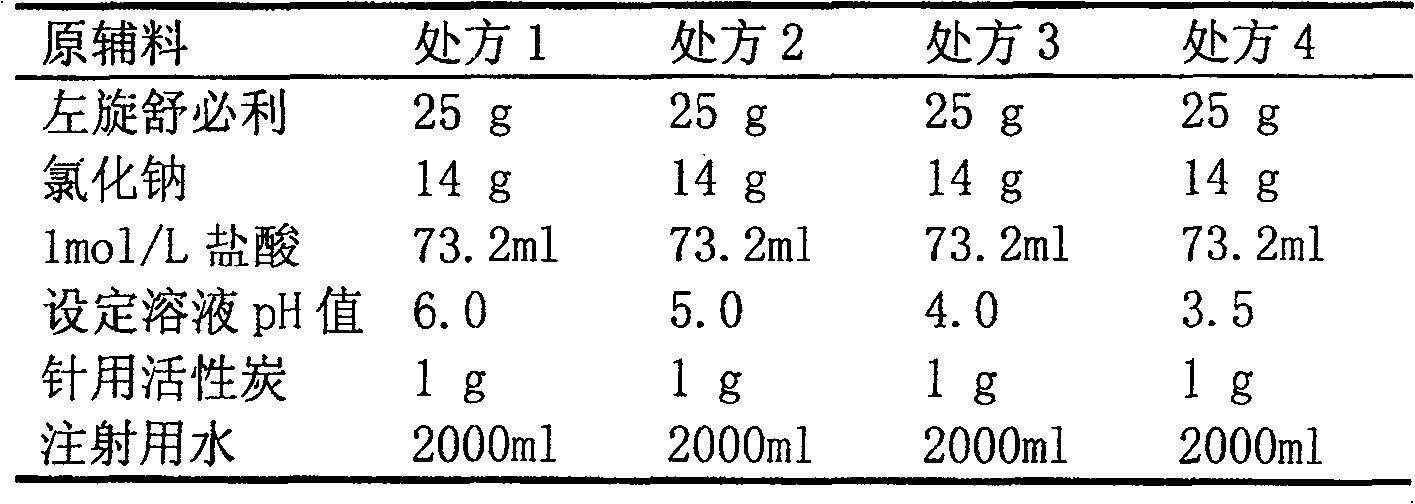
[0017] The preparation of embodiment 1 levosulpiride small volume sodium chloride injection
[0018] prescription composition
[0019]
[0020] Preparation:
[0021] (1) Take the prescribed amount of levosulpiride, 1mol / L hydrochloric acid, and sodium chloride and add them to about 1500ml of water for injection. After levosulpiride is completely dissolved, add water for injection to 2000ml, and use 0.1M hydrochloric acid and 0.1M sodium hydroxide solution Adjust the pH value to the set value;
[0022] (2) Add the gac of prescription quantity and stir and adsorb for 15 minutes, after rough filtration with sand rod, fine filter with the microporous membrane of 0.45um, check the clarity of medicinal liquid;
[0023] (3) Fill and seal the medicinal solution in ampoules, 2ml / support;
[0024] (4) Steam sterilization at 115°C for 30 minutes, leak detection, light inspection, printing and packaging.
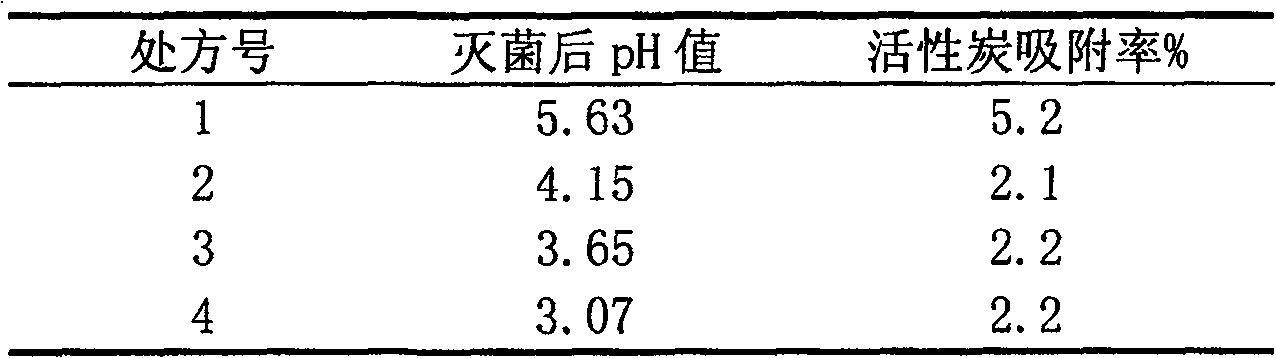
[0025] The injections prepared by each prescription are colorless and clear ...
Embodiment 2
[0029] The preparation of embodiment 2 levosulpiride sodium chloride infusion
[0030] Prescription: Levosulpiride 25.0g
[0031] 1mol / L sulfuric acid 73.2ml
[0032] Sodium chloride 900.0g
[0033] Water for injection 100000ml
[0034] A total of 1000 bottles were made
[0035] Preparation:
[0036] (1) Take levosulpiride and 1mol / L hydrochloric acid according to the prescription amount, add them to about 4500ml water for injection, stir to dissolve them completely, then add the solution to about 250000ml water for injection, stir and mix evenly;
[0037] (2) Add the sodium chloride of recipe quantity, stir and make dissolving;
[0038] (3) adjust the pH to 4.5 with hydrochloric acid or sodium hydroxide of 0.1mol / L;
[0039] (4) Add 0.03% (w / v) activated carbon and stir for adsorption for 15 minutes, remove the activated carbon by filtration, and measure the content of levosulpiride;
[0040] (5) Add water for injection to the full amount;
[0...
Embodiment 3
[0043] The preparation of embodiment 3 levosulpiride freeze-dried powder injection
[0044] Prescription: Levosulpiride 25.0g
[0045] 1mol / L benzenesulfonic acid 73.2ml
[0046] Mannitol 250.0g
[0047] Water for injection 1000ml
[0048] Activated carbon 0.5g
[0049] A total of 1000 bottles were made
[0050] Preparation:
[0051] (1) Take the prescribed amount of levosulpiride and add it to about 800ml of water for injection, add the prescribed amount of 1mol / L methanesulfonic acid, stir to dissolve it completely, add the prescribed amount of mannitol, add water for injection to 1000ml, stir and mix evenly , add 1M hydrochloric acid or sodium hydroxide to adjust the pH value to 4.5±0.5;
[0052] (2) add the gac of recipe quantity, after sand stick coarse filtration, by fine filtration with the microporous membrane of 0.45um, check the clarity of medicinal liquid;
[0053] (3) The medicinal liquid is divided into vials, 1ml / bottle;
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com