Recombinant adenovirus lyophilized preparation and preparing method thereof
A technology for recombinant adenovirus and freeze-dried preparations, which is applied in the field of recombinant adenovirus freeze-dried preparations and preparations, to achieve the effects of long validity period, good safety and stability, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
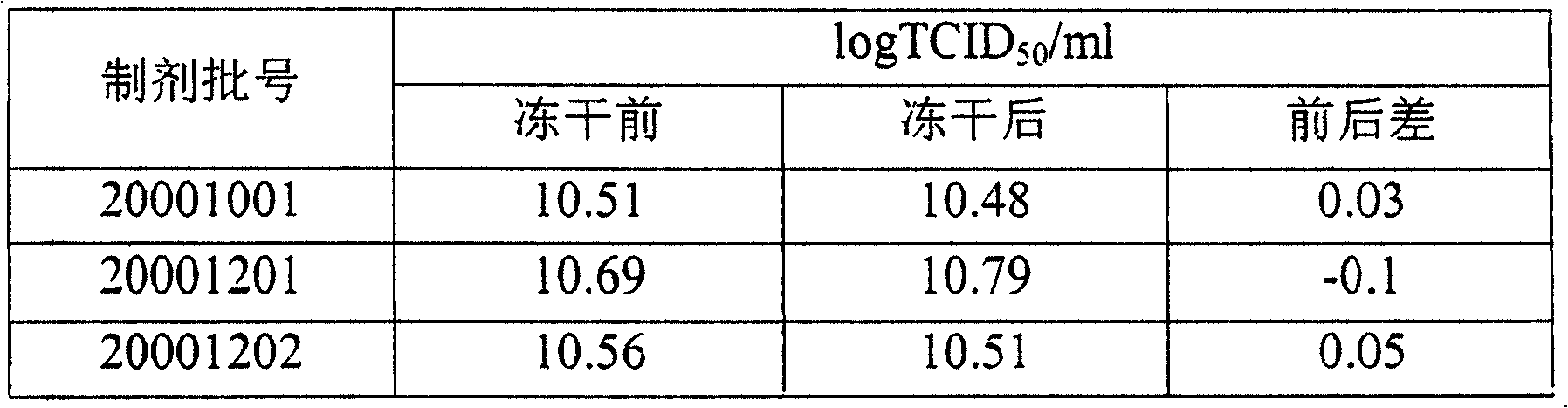
[0020] The recombinant adenovirus preparation containing the lyoprotectant was prepared according to the amount of adenovirus contained in it at 0.5×10 12 The concentration of the number of particles / ml is divided into 1ml / bottles, and a part of the preparation is stored at 4°C with a stopper and a cap for the detection of the infectious titer before the preparation is freeze-dried. Freeze dried. 3 different batches of recombinant adenovirus preparations containing lyoprotectants were freeze-dried, and the infectious titer test proved that: the freeze-dried recombinant adenovirus preparations made by the method of the present invention had higher infectious titers than frozen preparations after freeze-drying. Only drop in 0.03-0.05logTCID before drying 50 / ml between. (Table 1)
[0021] Table 1: Infectious titer determination of recombinant adenovirus preparations before and after freeze-drying
[0022]
Embodiment 2
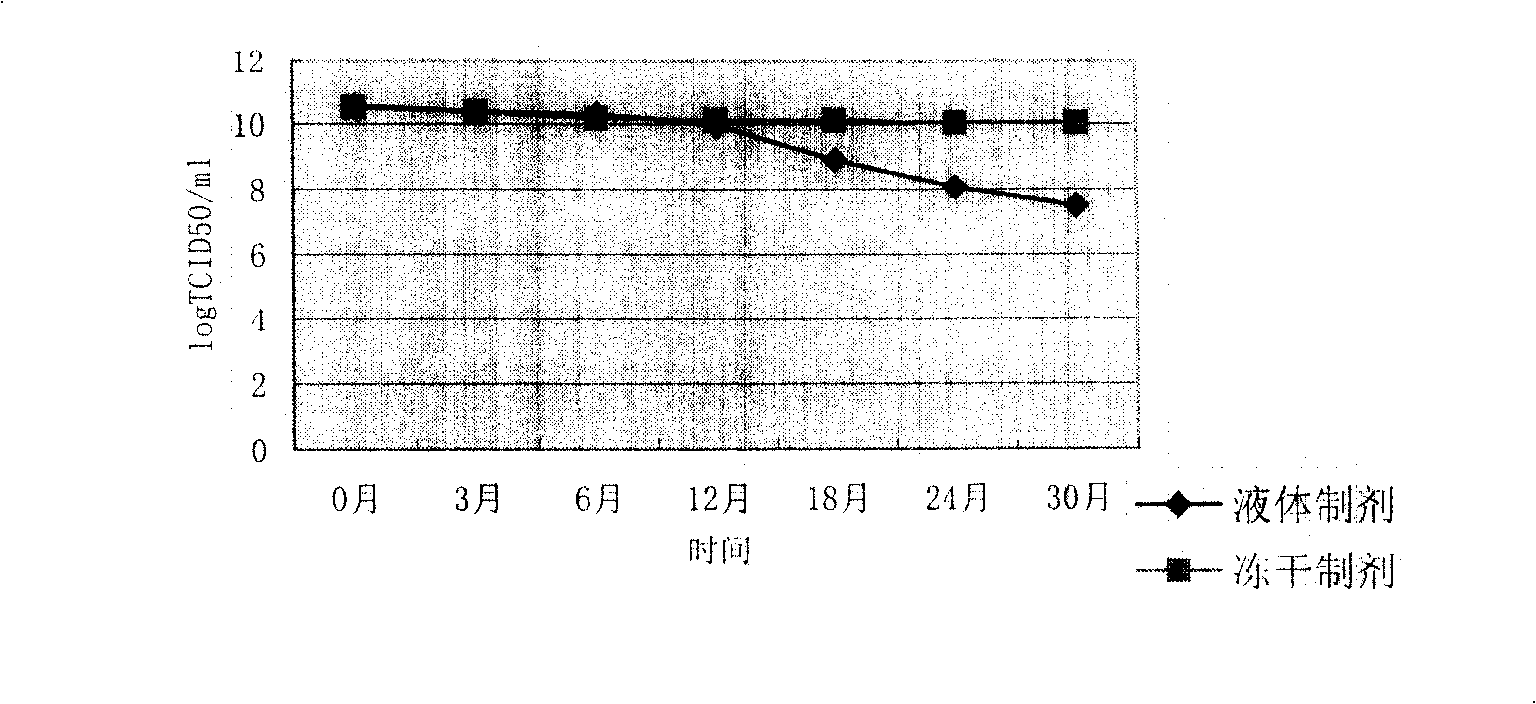
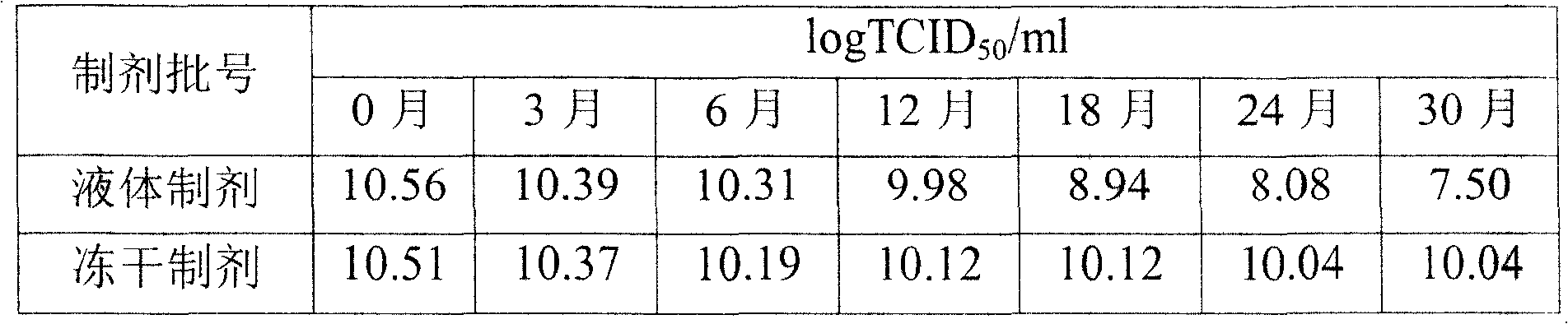
[0024] The same batch of recombinant glandular glands containing sucrose, mannitol, gelatin, L-cysteine, trehalose, potassium chloride, potassium dihydrogen phosphate, basic amino acid medium (Dulbecco's Modified Eagle Medium, D-MEM for short) balanced salt The virus freeze-dried preparation and the liquid control preparation without freeze-drying were stored in the same 4-8°C refrigerator, and the infectivity titer of the preparation was measured by random random sampling. The results proved that: the freeze-dried recombinant adenovirus prepared by the method of the present invention The preparation has good stability. When stored at 4-8°C for 30 months, the infectious titer of the preparation drops to 0.5logTCID 50 / ml, and the liquid control preparation without freeze-drying was stored at 4-8°C for 30 months, and the infectious titer decreased significantly, reaching 3logTCID 50 / ml. This result proves that: the infectivity titer of the non-lyophilized liquid control prepa...
Embodiment 3
[0028] Lyoprotectant containing sucrose, mannitol, gelatin, L-cysteine, trehalose, potassium chloride, potassium dihydrogen phosphate and basic amino acid medium (Dulbecco's Modified Eagle Medium, referred to as D-MEM) balanced salt Recombinant adenovirus preparation and lyoprotectant and basic amino acid medium (Dulbecco's Modified Eagle Medium, referred to as D- MEM) balanced salt solution, and the recombinant adenovirus preparation diluted with PBS were freeze-dried under the same vacuum freeze-drying condition, and then randomly sampled to determine the infectious titer of the preparation. The results proved that the virus activity was basically lost after freeze-drying the preparation without protective agent. (table 3)
[0029] Table 3: Effects of lyoprotectants on infectivity titers of recombinant adenovirus lyophilized preparations
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com