Cyclosporine microball preparation for treating endophthalmitis
A technology of cyclosporine and microspheres, which is applied in the direction of cyclic peptide components, bulk delivery, drug combination, etc., can solve the problems of recurrent or chronic inflammatory reactions, poor intraocular permeability, easy recurrence, etc., and achieve Overcome the limitation of the blood-ocular barrier, good therapeutic effect, no toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
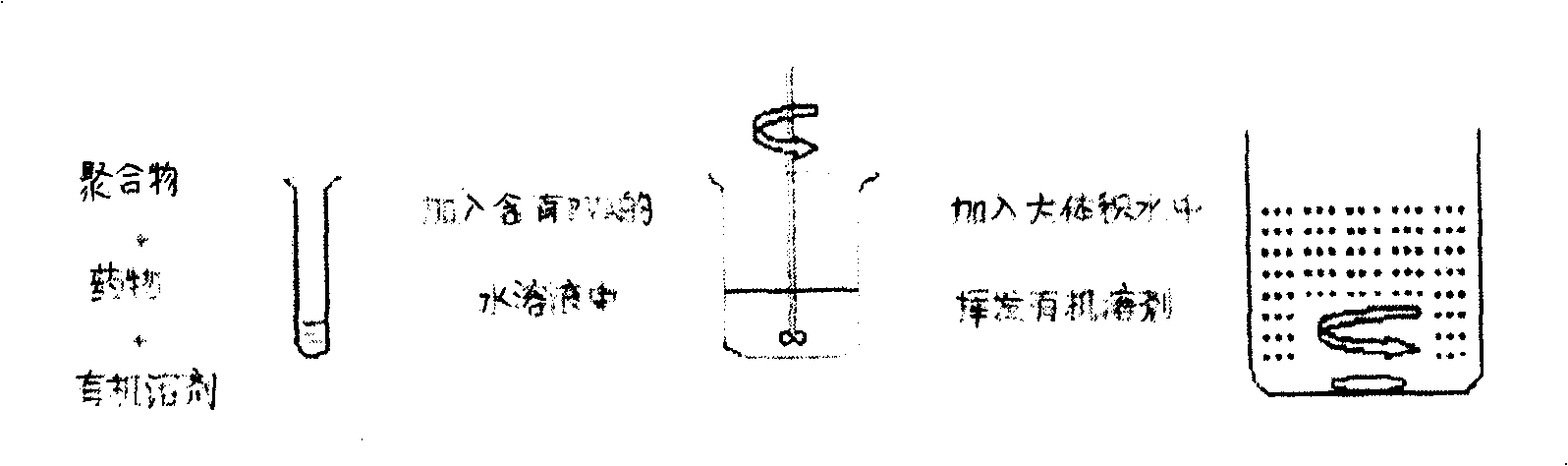
[0054] At room temperature, add cyclosporin A and PLGA (75 / 25, MW=15000) into a stoppered test tube and dissolve in dichloromethane. After fully dissolving, pour the organic phase into the water phase stirred at a certain speed In, and continue stirring at this speed for 6min (800rpm). Then the system was dispersed into 200 ml aqueous solution containing PVA (polyvinyl alcohol, concentration 0.1%), and was volatilized for 5 hours under magnetic stirring at room temperature to remove dichloromethane to solidify the microspheres. Centrifuge at 4000rpm at 15°C for 15min. Collect the supernatant to obtain microsphere suspension. The obtained microspheres were stored freeze-dried. For clinical use, use water for injection, normal saline for injection or glucose solution for injection to make a microsphere suspension, and inject it into the vitreous of the eyeball.
Embodiment 2
[0056] The preparation method of cyclosporin A microsphere
[0057] At room temperature, add cyclosporine A and PLGA (75 / 25, MW=15000) (10% ratio of drug to carrier material, W / W) in dichloromethane in a stoppered test tube, and after fully dissolving , the organic phase was quickly poured into the aqueous phase stirred at a certain speed, and continued to stir at this speed for 6min (stirring speed 800rpm). Then this system was dispersed in 200ml aqueous solution containing PVA (polyvinyl alcohol, concentration 5%), and under magnetic stirring at room temperature, dichloromethane was volatilized and removed to solidify the microspheres. Centrifuge at 4000rpm at 15°C for 15min. Collect the supernatant to obtain microsphere suspension. The obtained microspheres were stored freeze-dried.
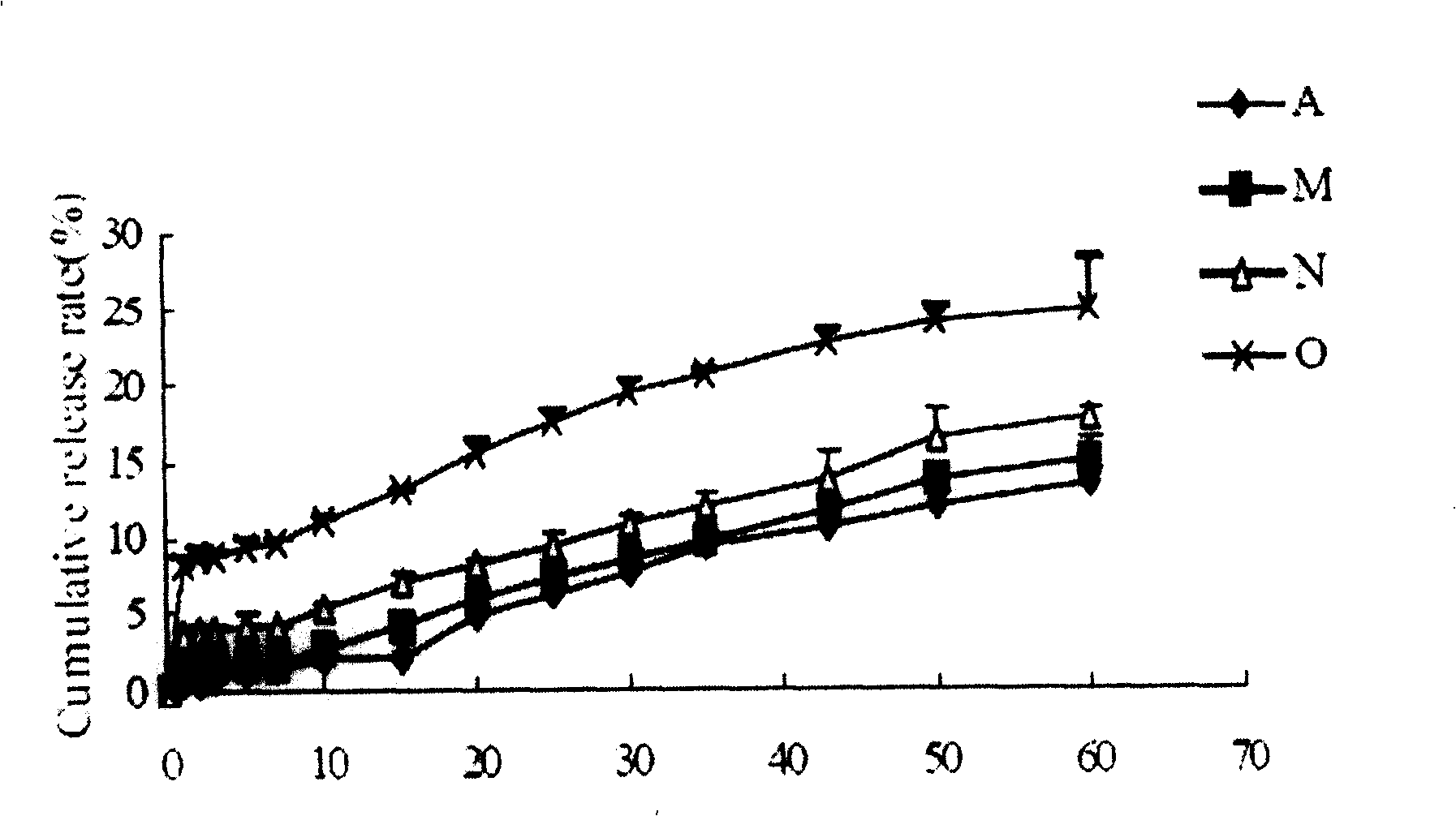
[0058] The prepared cyclosporine-loaded microspheres have a particle size distribution range of 10-70 μm, and the microsphere particles are observed by a scanning electron microscope to sho...
Embodiment 3
[0059] Embodiment 3, the preparation method of carrying cyclosporin A microsphere
[0060] At room temperature, add cyclosporin A and PLGA (75 / 25, MW=15000) (drug to carrier material ratio 20%, W / W) and pluronic F68 (0.5%) co-dissolved in a stoppered test tube In dichloromethane, after fully dissolving, the organic phase was quickly poured into the aqueous phase stirred at a certain speed, and continued to stir at this speed for 6 minutes (stirring speed 1000rpm). Then the system was dispersed into 200 ml aqueous solution containing PVA (polyvinyl alcohol, concentration 0.5%), and under magnetic stirring at room temperature, dichloromethane was volatilized and removed to solidify the microspheres. Centrifuge at 4000rpm at 15°C for 15min. Collect the supernatant to obtain microsphere suspension. The obtained microspheres were stored freeze-dried.
[0061] The prepared cyclosporine-loaded microspheres have a particle size distribution range of 10-70 μm, and the microsphere pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com