Double-chain RNA and method for initiate, enhance or adjust immunomodulatory of immune response
A technology of antigen and composition, applied in the field of non-coding RNA motifs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0007] Various embodiments of the invention include:
[0008] CLAIMS 1. A method of enhancing an immune response to an antigen comprising administering a composition consisting of double-stranded RNA and co-administering said composition with said antigen.
[0009] 2. A method of enhancing or modulating an immune response to an antigen, when said antigen is already present in the body, comprising administering a composition consisting of double stranded RNA.
[0010] 3. The method of paragraph 1, wherein the RNA is a non-coding RNA.
[0011] 4. The method of paragraph 1 or 2, wherein the double-stranded RNA consists of polyadenine and polyuracil.
[0012] 5. The method of paragraph 1 or 2, wherein the double-stranded RNA consists of one of polyguanine and polycytosine or polyinosine and polycytosine.
[0013] 6. The method of paragraph 1 or 2, wherein the double-stranded RNA consists of adenine and uracil.
[0014] 7. The method of paragraph 1 or 2, wherein the double-stran...
Embodiment 1
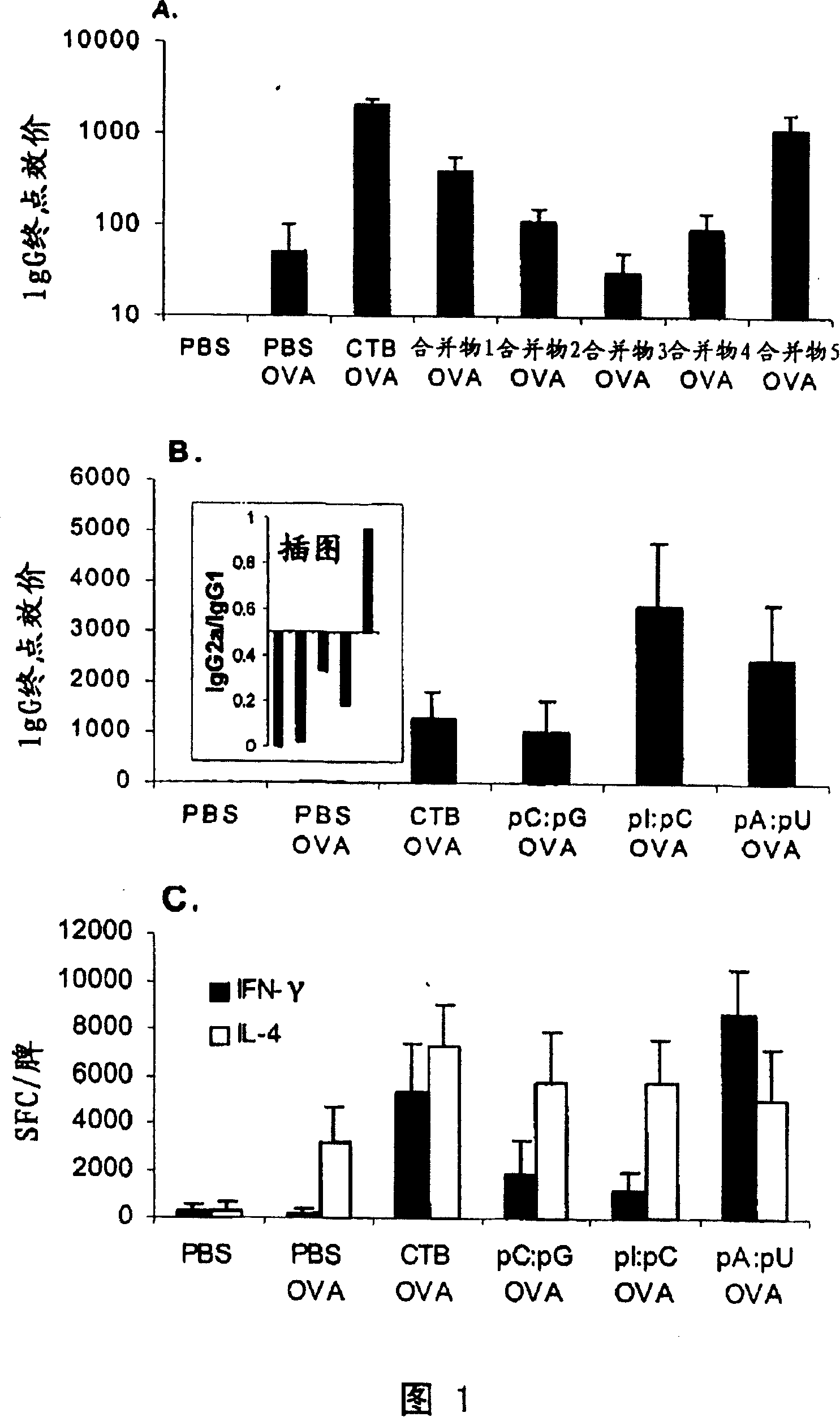
[0195] Example 1: Effects of Various Mergers of RNA Motifs on Antibody Responses to Standard Antigens (OVA)
[0196] The effect of various RNA pools (Table 1 ) on adaptive immunity was determined in C57B1 / 6 mice co-immunized with OVA via the airway. As described in the "Materials and Methods" section, the antibody responses were expressed as mean ± SEM of IgG endpoint titers (n=4 / group). As controls, OVA in sterile PBS, OVA with cholera toxin subunit B (CTB) and PBS alone were used, respectively. As shown in Figure 1A, the pool of dsRNAs corresponding to the greatest impact on the antibody response had significantly enhanced specific immunity. In addition, pools of single-stranded species that are complementary to each other reproduce this enhancement.
[0197] This suggests that both the residue nature and the secondary structure of RNAs determine their ability to function as "danger" motifs, influencing specific B cell responses.
Embodiment 2
[0198] Example 2: Effect of various individual dsRNA motifs on induction of antibody responses against OVA
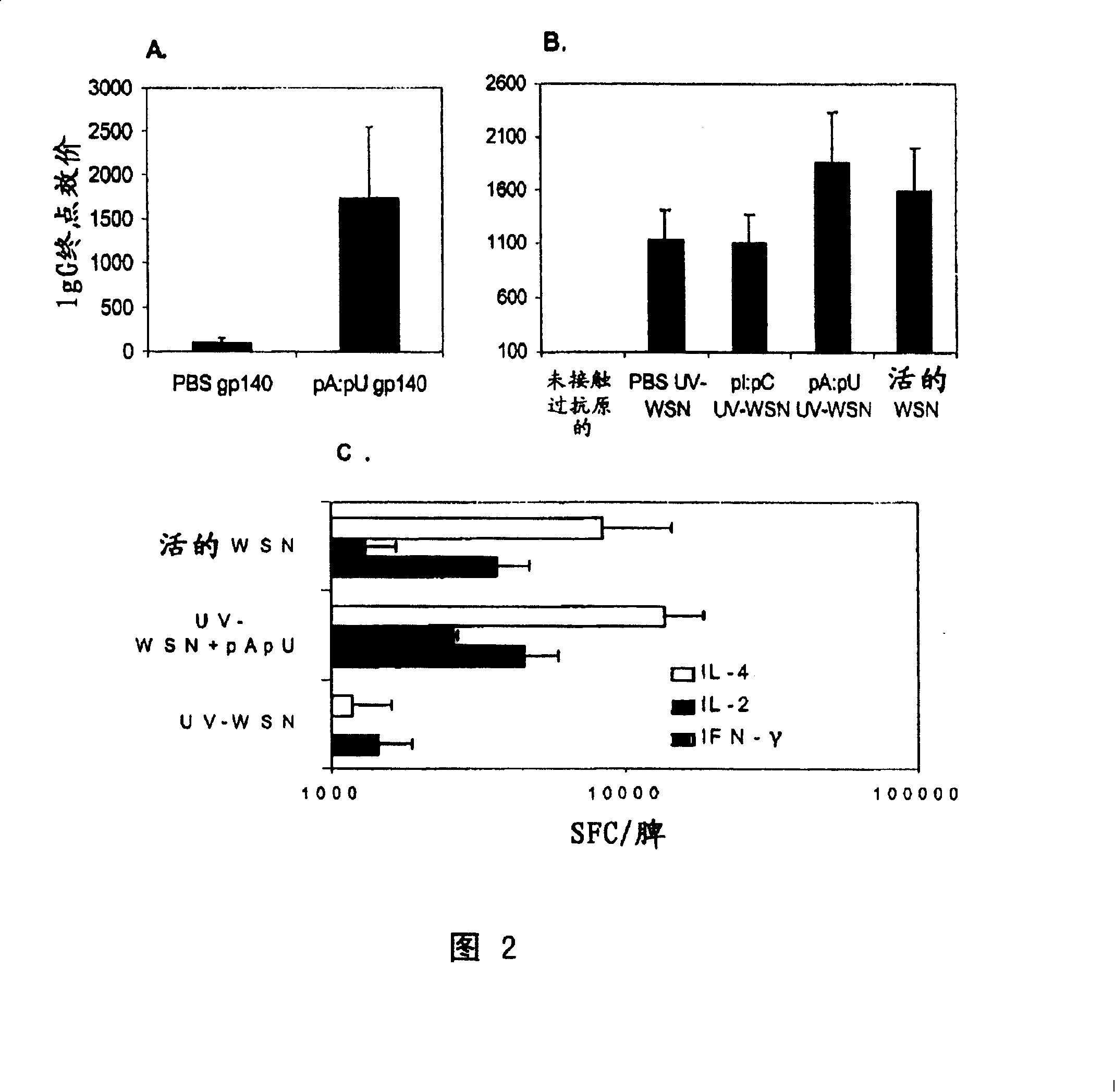
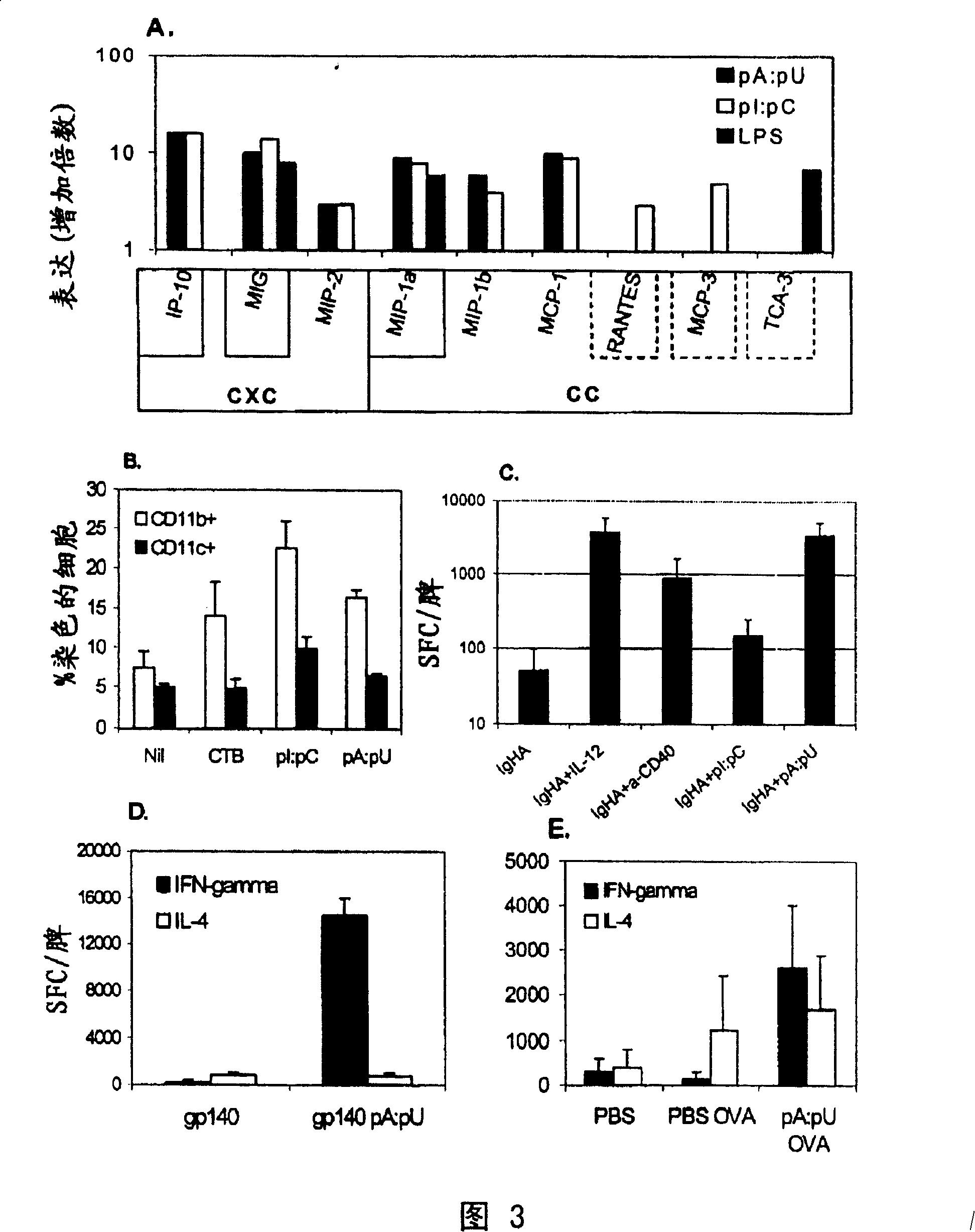
[0199]Experiments were performed as described in the previous examples and in the "Materials and Methods" section, however, instead of using pools of RNAs, we used individual dsRNAs. The results are shown in Figure 1B in the same manner as Figure 1A. The results are representative of two independent experiments. Inset: ratio of mean IgG2a and IgG1 titers to OVA. The order from left to right is similar to the main figure: PBS OVA, CTB OVA, pC:pG OVA, pI:pC OVA and pA:pU OVA. In the second round of screening shown in Figure 1B, two types of motifs constituting double-stranded RNA, pA:pU and pI:pC, were confirmed to contribute to the generation of IgG responses against specific antigens in C57BL / 6 mice have a noticeable impact. Similar results were obtained in TLR4- / -C3H / HeJ mice with impaired responses to endotoxin (not shown).
[0200] Thus, individual RNA motifs ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com