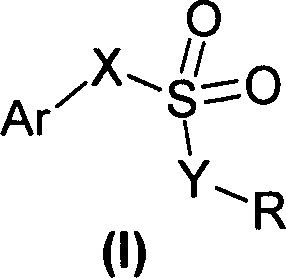
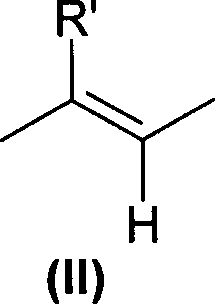
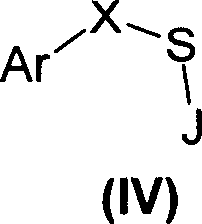
Sulfonic acids, their derivatives and pharmaceutical compositions containing them
A technology of sulfonic acids and compounds, applied in the field of sulfonic acids and their derivatives and pharmaceutical compositions containing them
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0163] Preparation of E-arylene sulfonic acid (sodium salt)
[0164] Arylethanesulfonic acid was dissolved in thionyl chloride (5 mL), and the resulting solution was refluxed overnight. After cooling at room temperature, thionyl chloride was evaporated in vacuo, and the crude arylethanesulfonyl chloride was diluted with dry THF (5 mL), cooled in an ice-water bath at 0°C; 1N NaOH aqueous solution (0.64 mmol) was added at 4°C; the ice-water bath was removed, The reaction mixture was left standing for about 1 hour, and the temperature was raised to room temperature, resulting in the precipitation of a white solid. The organic sodium salt was vacuum filtered, washed with THF, and dried in a vacuum oven at 40° C. to obtain pure E-arylene sulfonic acid sodium salt (0.32-0.51 mmol) as a white powder (50-80% yield ).
[0165] Following the method described above, the following compounds were prepared:
[0166] E-2-(4-isobutylphenyl)ethylenesulfonic acid sodium salt (10)
[0167]...
Embodiment 3
[0175] A General Synthesis Method for E-Arylethene Sulfonamides
[0176] Arylethanesulfonic acid (0.64 mmol) was dissolved in thionyl chloride (5 mL), and the resulting solution was refluxed overnight. After cooling at room temperature, thionyl chloride was evaporated in vacuo, and the crude arylethanesulfonyl chloride was diluted with dry THF (5 mL), cooled in an ice-water bath at 0 °C; the selected amine (1.28 mmol) was added dropwise. The ice-water bath was removed, and the reaction mixture was allowed to stand until the temperature rose to room temperature. After complete disappearance of the starting reagent, the solvent was evaporated in vacuo, and chloroform (10 mL) and water (10 mL) were added to the residue; the two phases were separated by shaking, and the organic phase was washed with water (3×15 mL), dried over sodium sulfate, and evaporated in vacuo , the crude product was obtained, which was purified by flash chromatography to isolate pure E / Z-arylethenesulfon...
Embodiment 4
[0214] A General Synthesis of Aryl Methanesulfonamides
[0215] (1-Methyl-5-isobutyrylpyrrolyl)-1-methanesulfonamide (30)
[0216] Starting from the commercially available reagent methyl 1-methyl-2-pyrrole acetate, it undergoes Friedel Crafts acylation with isobutyryl chloride to give (1-methyl-5-isobutyrylpyrrolyl)-1- formyl acetate, thus synthesizing the compound. The ester group is then hydrolyzed. According to the experimental procedure described in WO02 / 0704095, the related sodium salt of (1-methyl-5-isobutyrylpyrrolyl)-1-methanesulfonate was obtained.
[0217] (1-Methyl-5-isobutyrylpyrrolyl)-1-methanesulfonic acid sodium salt (0.64 mmol) was dissolved in thionyl chloride (5 mL), and the resulting solution was refluxed overnight. After cooling at room temperature, thionyl chloride was evaporated in vacuo, and the crude (1-methyl-5-isobutyrylpyrrolyl)-1-methanesulfonyl chloride was diluted with dry THF (5 mL), cooled in an ice-water bath at 0°C; dropwise into Ammonia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com