Artificial synthesized insulin-simulated peptide and its application
A technology of artificial synthesis and insulin, applied in the direction of medical preparations containing active ingredients, peptides, drug combinations, etc., can solve the problems of high synthesis cost and difficulty in synthesis, and achieve the effect of low preparation cost, high product purity and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: Using anti-insulin polyclonal antibody as target protein, screening phage 9 peptide library by phage display method (Ph.D.-C7C TM ) to obtain positive phage clones
[0015] The phage display method uses genetic engineering to clone a set of synthetic oligonucleotide fragments of a certain length and random sequence into a specific expression vector, so that the expression product is presented on the surface of filamentous phage in the form of fusion protein. Since the peptide library contains all possible amino acid sequences of small peptides of this length or most of them, each phage presents one of the peptides, so the library has a large capacity and is easy to screen and amplify. Infect E. coli with the phage peptide library, and the random oligonucleotide fragments recombined into the phage are replicated in E. coli and expressed in the coat protein of the phage. Then, coat the target protein on the microtiter plate. After mixing the phage peptide lib...
Embodiment 2
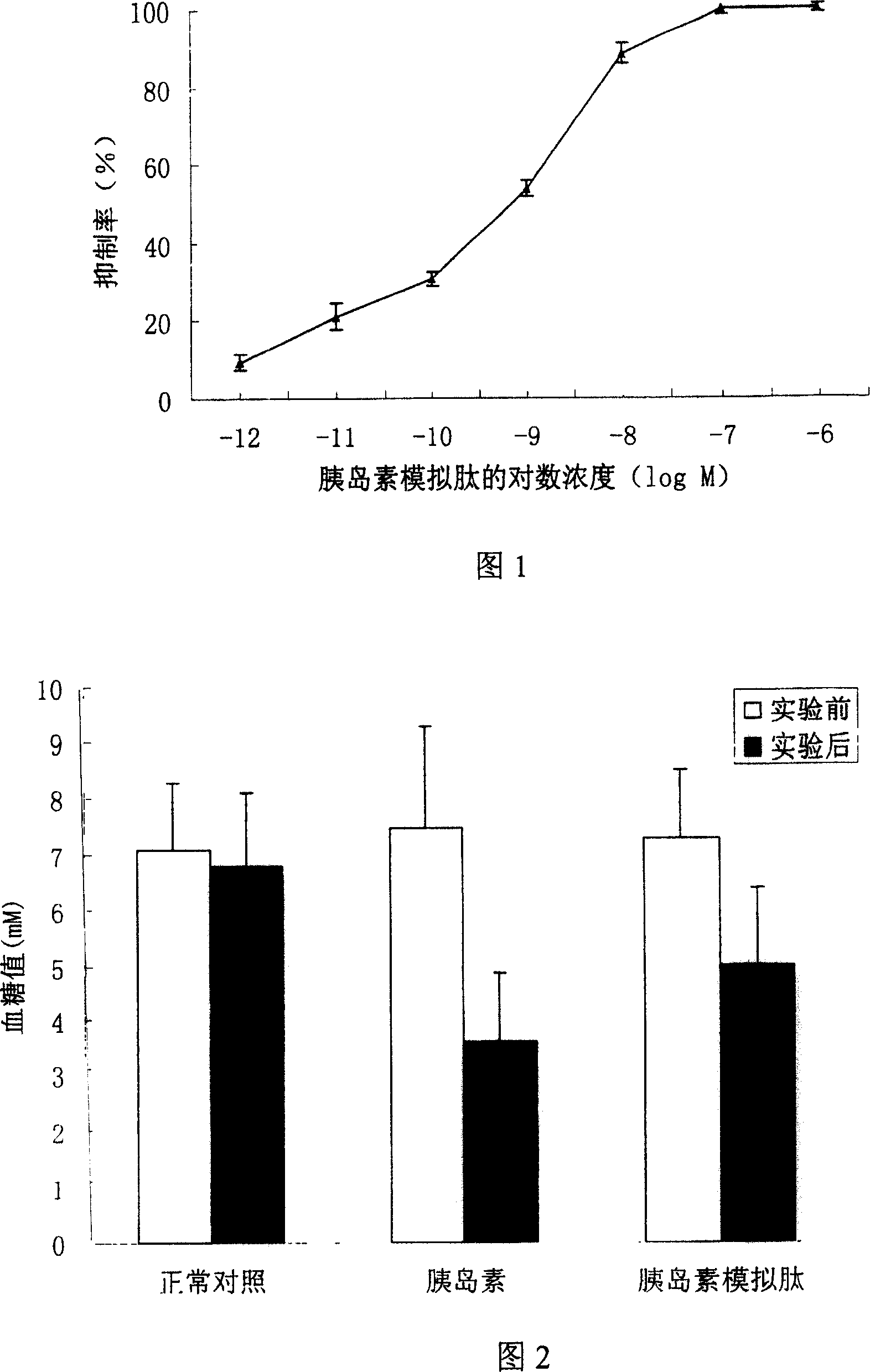
[0018] Embodiment 2: Cell competitive ELISA test
[0019] Test method: HepG2 cells were cultured to the logarithmic growth phase, and the same amount was inoculated into 96-well cell culture plates. After 24 hours, wash twice with PBS, fix with 10% Formalin-PBS for 15min, wash three times with PBS, and wash with 3% H 2 o 2 Treat for 10min. Wash 3 times with PBS, block with 2% BSA for 1 hour at 37°C, wash 3 times with PBST, add 10 -9 Insulin M, acted at 37°C for 90 minutes. After washing with PBST for 3 times, small peptides of different concentrations were added, and the cells were competed at 37°C for 90 minutes. Wash 3 times with PBST, incubate with primary antibody at 37°C for 1 hour, wash with PBST 5 times, add secondary antibody and incubate at 37°C for 1 hour. After washing 5 times with PBST and 1 time with PBS, the color was developed, and the absorbance (OD) at a wavelength of 450 nm was read by a microplate reader. 450 ). In the experiment, blank wells and posi...
Embodiment 3
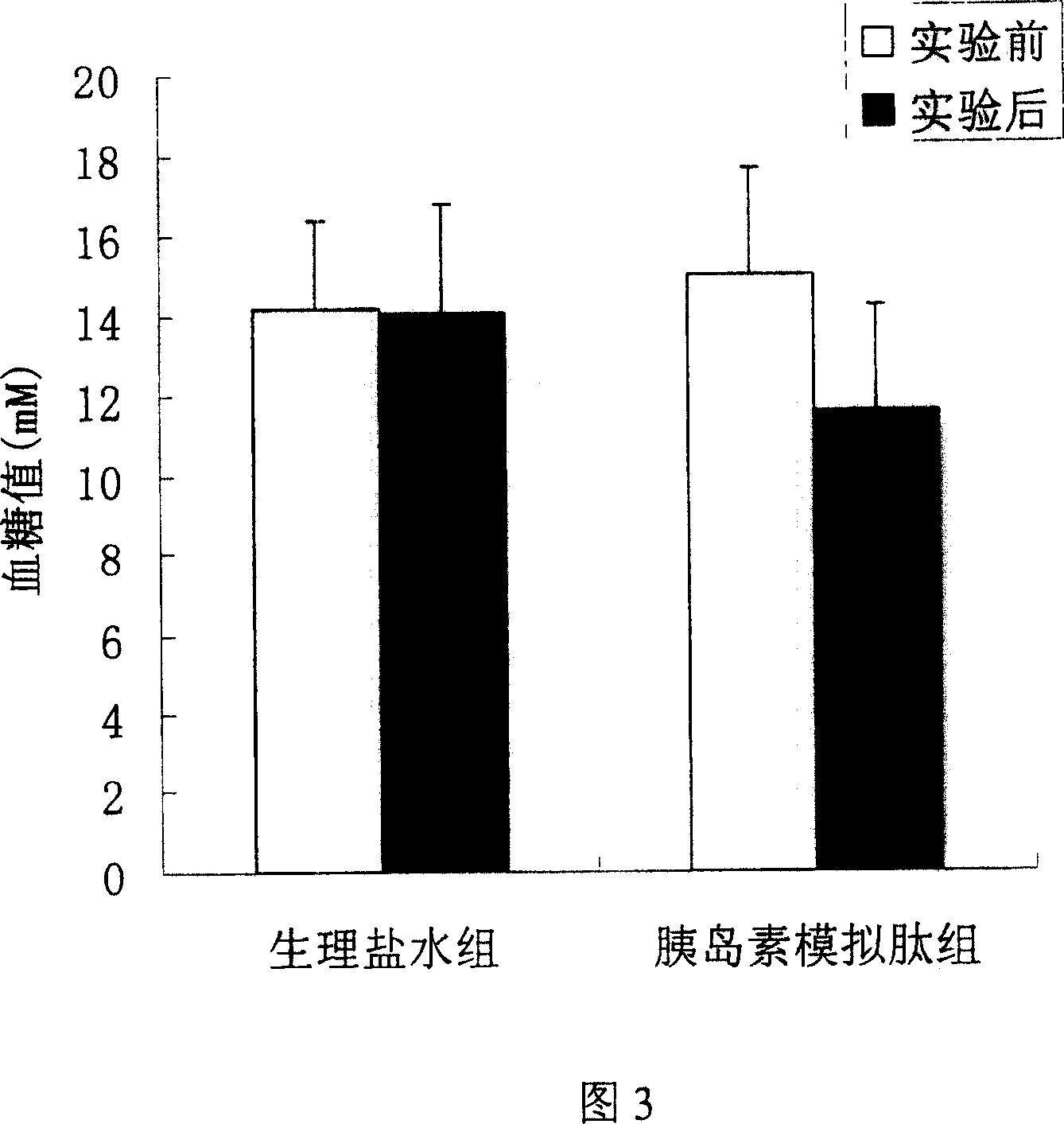
[0021] Example 3: Effects of Mimic Peptides on Blood Glucose in Normal ICR Mice
[0022] Test method: 30 normal ICR mice were randomly divided into normal control group (10 mice), insulin control group (10 mice) and insulin mimetic peptide treatment group (10 mice). The mice in each experimental group were marked separately, and after fasting for 12 hours, the fasting blood glucose was measured. The mice in the insulin mimetic peptide treatment group were injected with 100 μl of 1 mg / ml peptide solution (prepared in 0.9% normal saline), and the insulin control group was injected with 2 U / kg. For insulin, the normal control group was injected with an equal volume of 0.9% normal saline. Blood was collected 45 minutes after the injection, the blood glucose was measured by the o-toluidine method, and the changes of blood glucose before and after the injection were compared.
[0023] Test results: the results are shown in Figure 2, which shows the effect of the insulin mimetic pep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com