Polymer/graphite oxide anticoagulant nano composite material and preparation method thereof
A composite material and polymer technology, which is applied in the field of polymer/graphite oxide nano anticoagulant composite material and its preparation, can solve the problems that nano powder is not easy to disperse evenly, the mechanical properties of materials are affected, and there are no literature and patent reports. , to achieve the effect of prolonged anticoagulant performance, good sustained release performance, good biocompatibility and blood compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
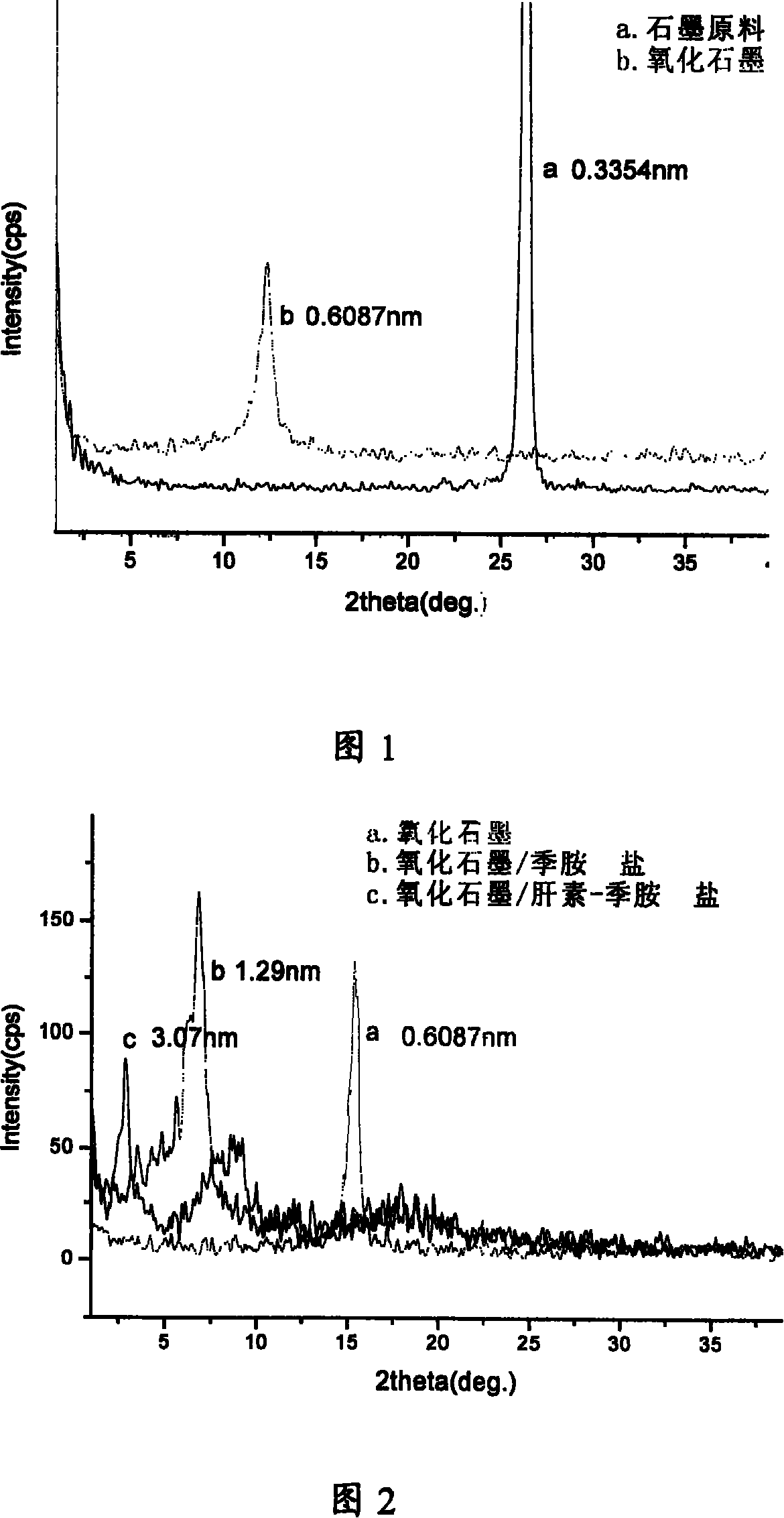
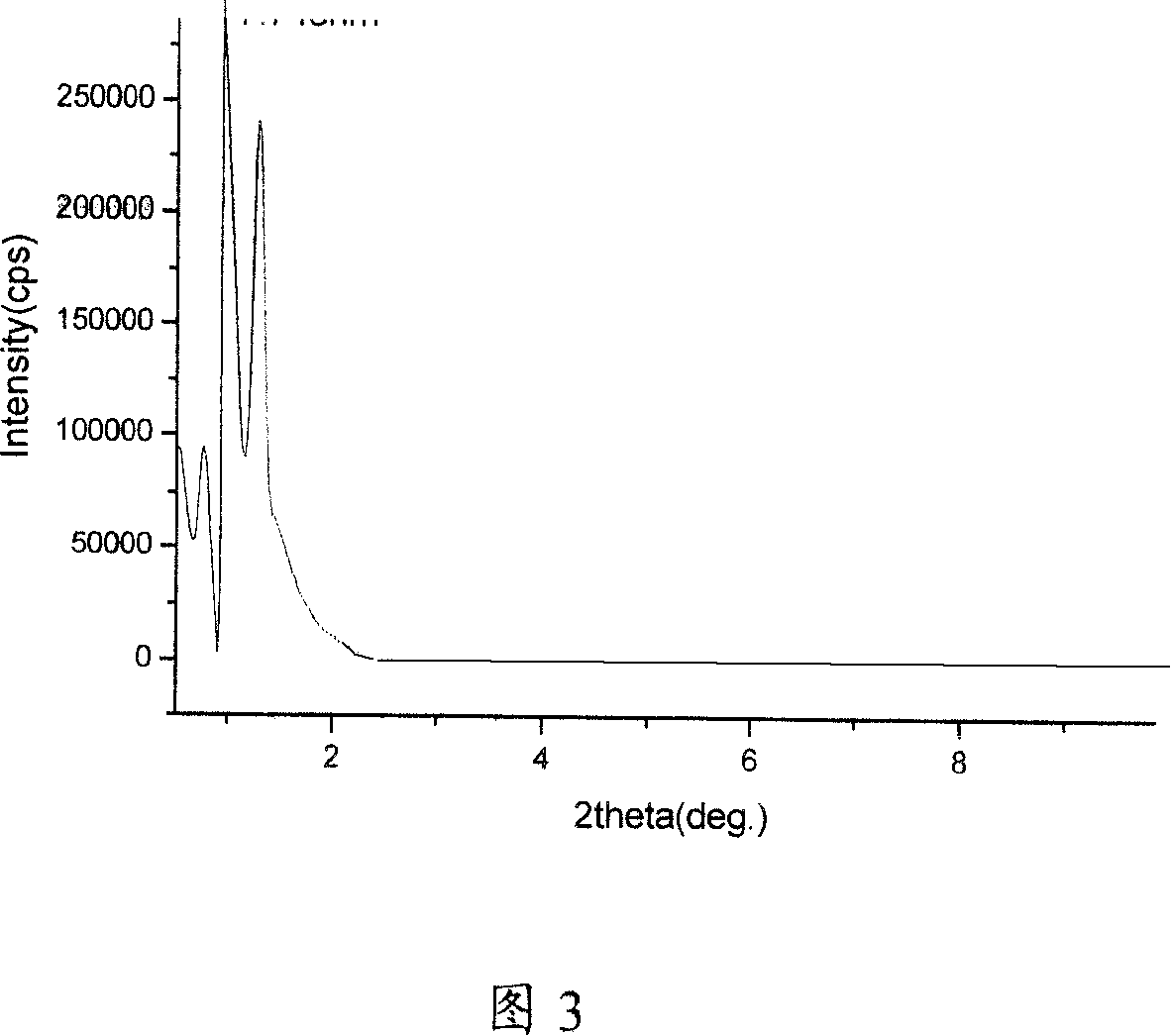
[0054] Embodiment 1, referring to Figures 1 to 3, and Figures 4-1 and 4-2:
[0055] Dissolve 0.01 part of graphite oxide in 2 mL of 0.1M NaOH solution (equivalent to 0.0002 parts of NaOH), and ultrasonically treat it for 20 minutes to obtain a black colloid; Add 0.3 parts of the ammonium complex to the graphite oxide colloidal solution treated with NaOH at 20-60°C, and ultrasonicate for 15 minutes to obtain a black precipitate; filter the precipitate and wash it with distilled water until no chloride ions can be detected (use 0.2% AgNO 3 solution detection); vacuum drying at 60°C for 24 hours, the resulting black solid is pulverized into a functional graphite oxide / heparin-quaternary ammonium salt anticoagulant complex;
[0056] Disperse 0.01 part of the above-mentioned functional graphite oxide / heparin-quaternary ammonium salt anticoagulant compound in 100 parts of dispersion medium toluene at 20-60 °C, add 100 parts of room temperature vulcanized silicone rubber, and stir vi...
Embodiment 2
[0058] Dissolve 10 parts of graphite oxide in 500 mL of 0.1 M NaOH (equivalent to 0.05 parts of NaOH) solution, and ultrasonically treat it for 2 hours to obtain a black colloid; Add 20 parts of the ammonium chloride complex into the graphite oxide colloid solution treated with NaOH at 20-60°C, and ultrasonicate for 30 minutes to obtain a black precipitate; filter the precipitate and wash it with distilled water until no chloride ions can be detected (with 0.2% AgNO 3 solution detection); vacuum drying at 60°C for 24 hours, the resulting black solid is pulverized into a functional graphite oxide / heparin-quaternary ammonium salt anticoagulant complex;
[0059] Disperse 10 parts of the above-mentioned functional graphite oxide / heparin-quaternary ammonium salt anticoagulant compound in 200 °C dispersion medium xylene at 20-60 °C, add 100 parts of room temperature vulcanized silicone rubber, and stir vigorously for 6 hours to make it Mix well, add 5 parts of anilinomethyltrimetho...
Embodiment 3
[0061] Dissolve 2 parts of graphite oxide in 60mL of 0.1M NaOH (equivalent to 0.006 parts of NaOH) solution, and ultrasonically treat it for 1 hour to obtain a black colloid; functional anticoagulant intercalation agent heparin sodium-octadecyltrimethyl Add 1 part of the ammonium chloride complex to the graphite oxide colloid solution treated with NaOH at 20-60°C, and ultrasonicate for 20 minutes to obtain a black precipitate; filter the precipitate and wash it with distilled water until no chloride ions can be detected (with 0.2% AgNO 3 solution detection); vacuum drying at 60°C for 24 hours, the resulting black solid is pulverized into a functional graphite oxide / heparin-quaternary ammonium salt anticoagulant complex;
[0062] Disperse 3 parts of the above-mentioned functional graphite oxide / heparin-quaternary ammonium salt anticoagulant compound and 0.5 part of the cross-linking agent peroxygen compound DCP in 100 parts of high-temperature vulcanized silicone rubber at 30 °...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com