Micro-molecular polypeptides capable of combining with gold staphylococcus virulence factor regulatory protein and its pharmaceutical use
A technology of virulence factor and protein regulation, which can be applied to peptide/protein components, medical preparations containing active ingredients, antibacterial drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1. T Genetic Engineering Expression and Isolation and Purification of RAP Protein
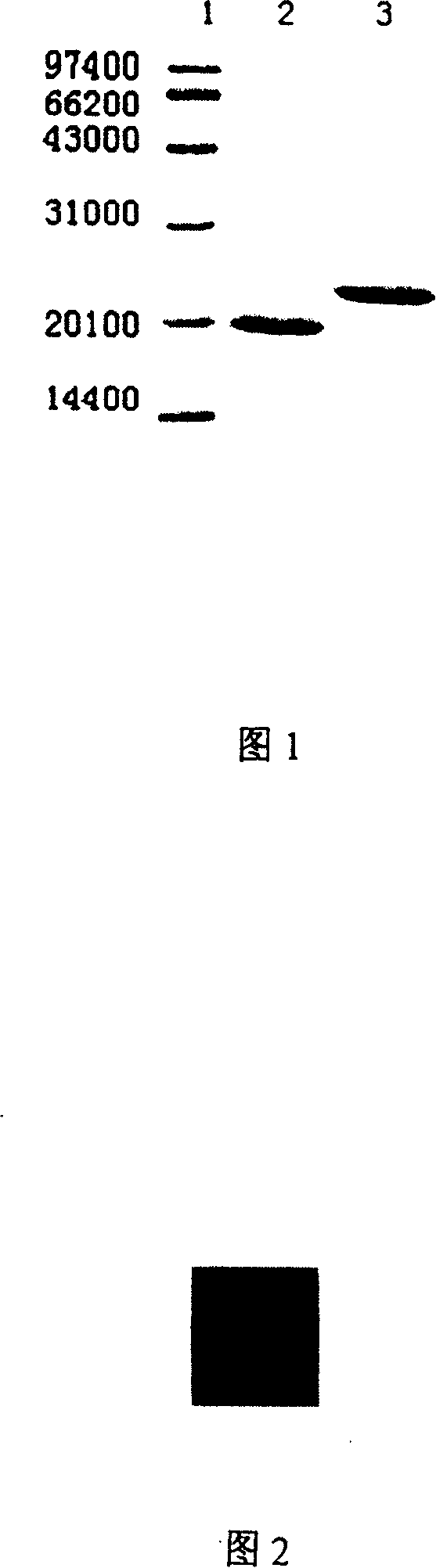
[0020] The correctly identified Escherichia coli TRAP gene was connected to the expression vector, and expressed in Escherichia coli. After SDS-PAGE electrophoresis, it was found that there were bands of the target protein in the supernatant and inclusion bodies, and the target protein in the supernatant accounted for the total More than 50% of the amount. The target protein with His tag was purified by metal chelate column, detected by SDS-PAGE, the molecular weight was about 23kD, and the purity was >90%. The His tag was cut off with thrombin, and the molecular weight of the purified TRAP protein was about 21kD, and the purity was >95%. (see accompanying drawing 1, wherein 1: molecular weight protein standard; 2: the TRAP protein of purification.).
Embodiment 2
[0021] Example 2. Identification of Purified TRAP Proteins
[0022] Through WESTEN BLOT detection, our purified TRAP protein can specifically react with the TRAP protein polyclonal antibody (Figure 2)
Embodiment 3
[0023] Example 3. Screening of TRAP-binding peptides
[0024] First, 100 μl of purified TRAP protein was used to coat the enzyme-linked plate, and placed at 4°C overnight. After blocking with 2% gelatin for 1 h, add the phage dodecapeptide library, incubate at room temperature for 1 h, wash the non-specifically bound phage with TBST (50 mmol / LTris-HCl, 0.1% TWEEN20, pH 7.5), and wash with 0.2 mmol / L Glycine-HCl pH2.2 elutes the specifically bound phage, and the eluate is neutralized with 1mmol / L Tris-HCl pH9.0. According to the method provided by the kit, the titer of the phage in the eluate was determined, and the titer of the eluted phage of the uncoated target protein was used as a control to determine the input-output ratio. At the same time, the eluted phages combined with TRAP were amplified and their titers were determined for the next round of screening. After 3 rounds of screening, the input and output of the assay has been significantly improved. The enriched ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com