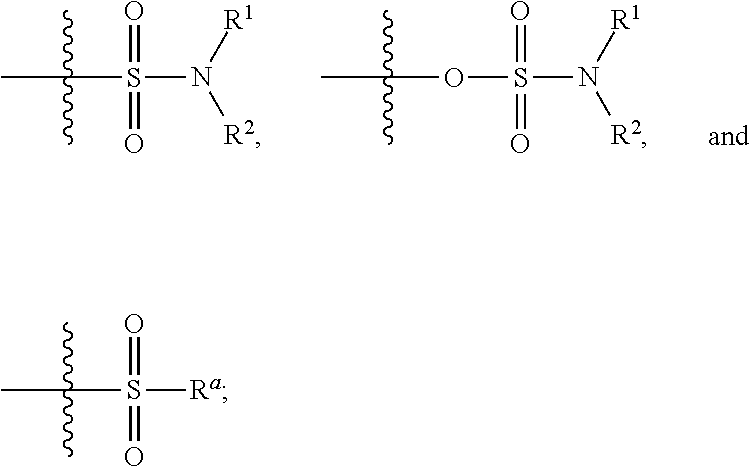
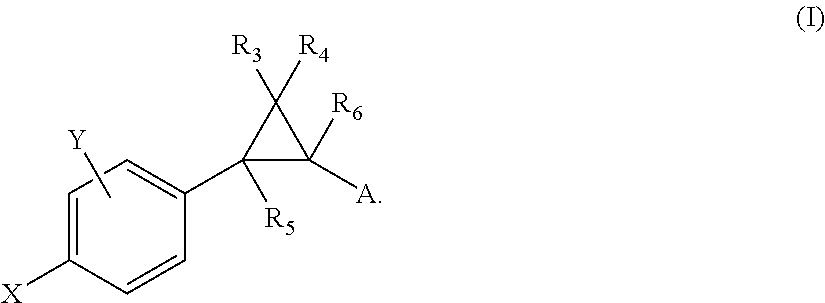
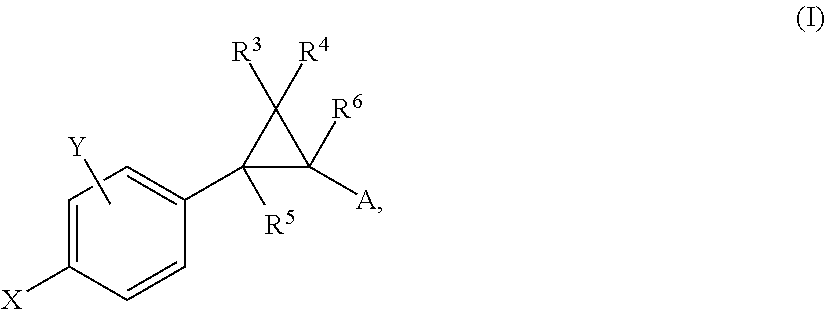
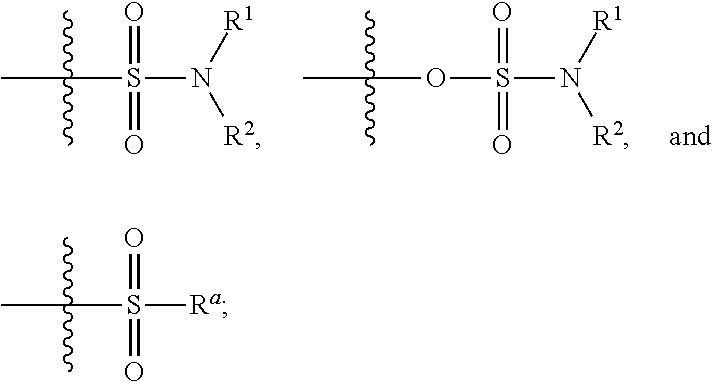
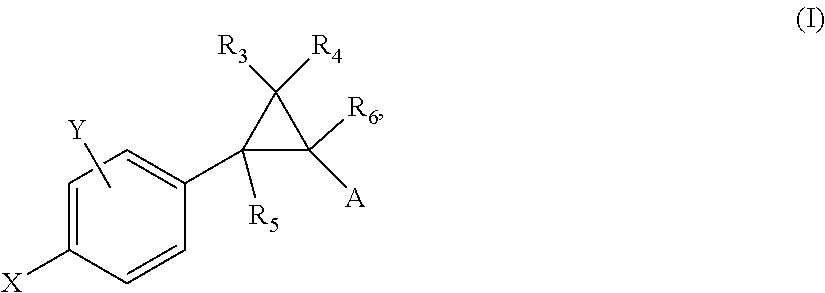
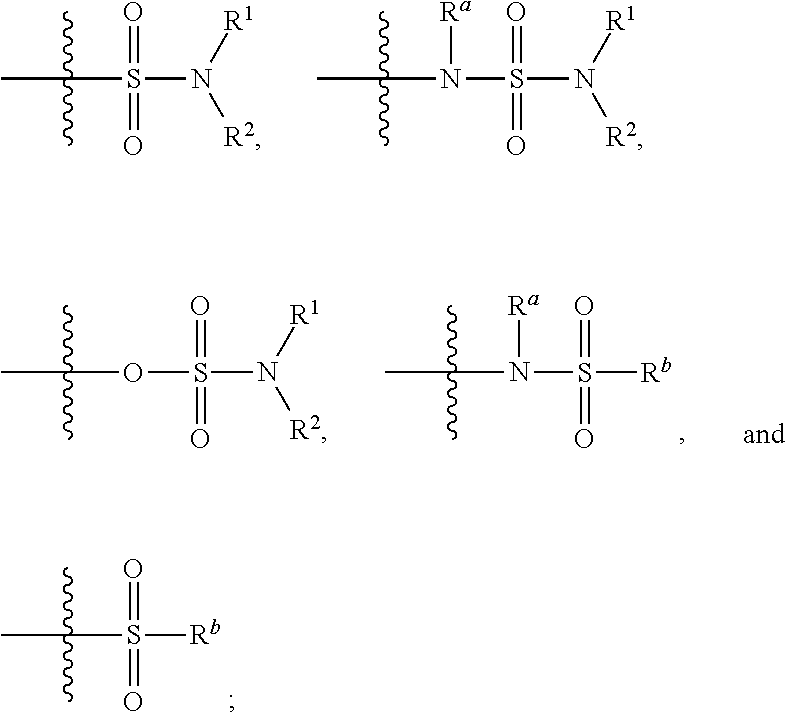
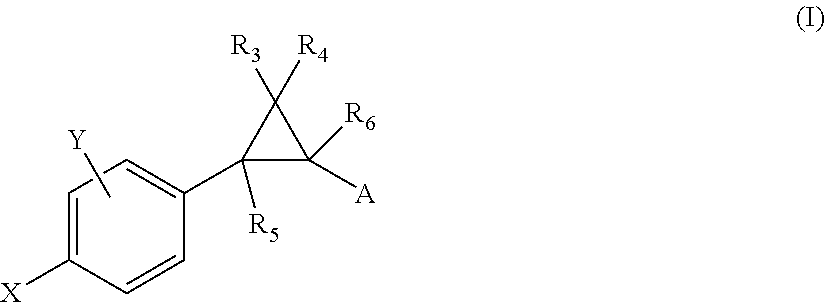
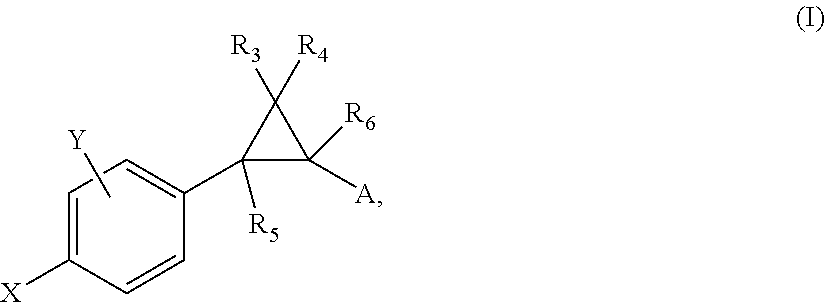
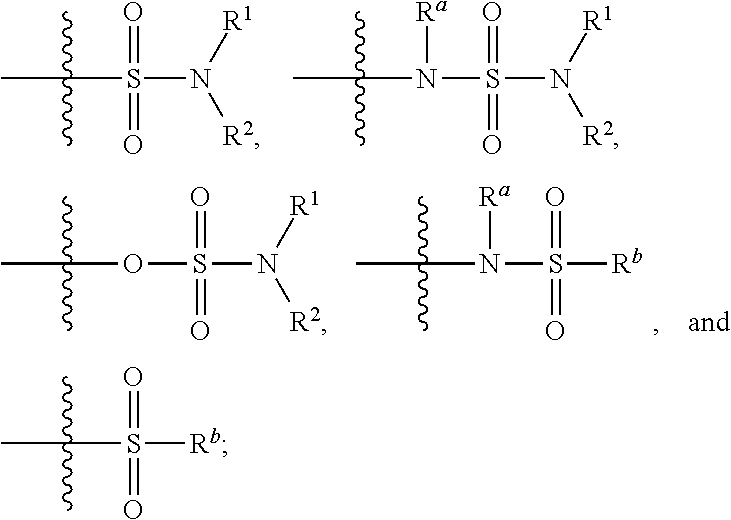
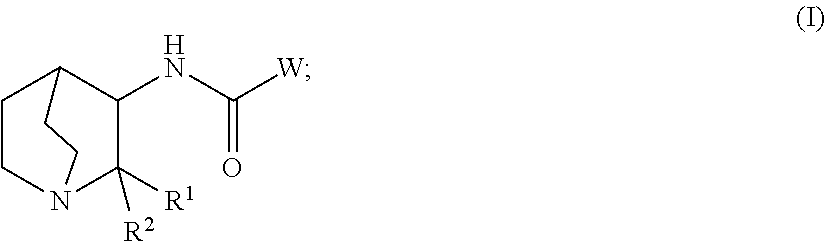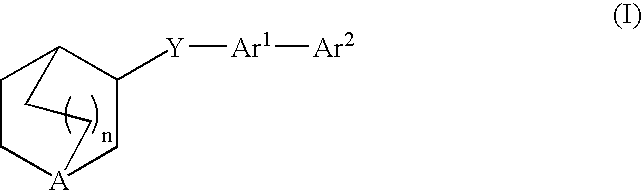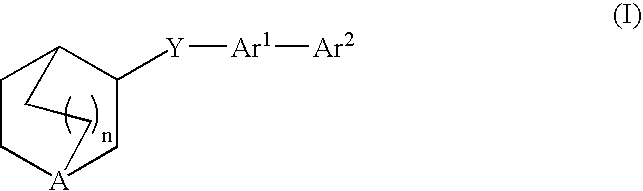Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Α7 nachr" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
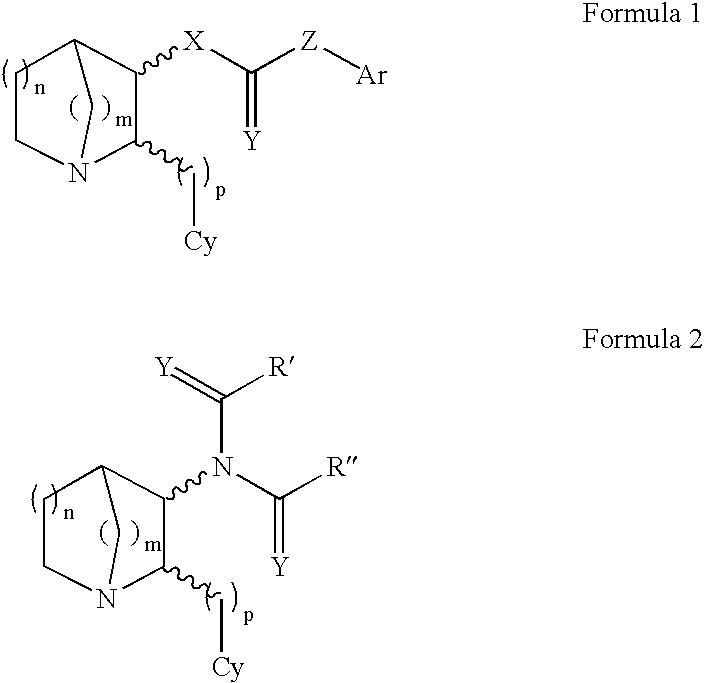
3-substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof
InactiveUS6953855B2Modulate activityWithout side effectAntibacterial agentsBiocideDiseaseThiocarbamate
The present invention relates to 3-substituted-2-(arylalkyl)-1-azabicycloalkanes, methods of preparing the compounds and methods of treatment using the compounds. The azabicycloalkanes generally are azabicycloheptanes, azabicyclooctanes, or azabicyclononanes. The aryl group in the arylalkyl moiety is a 5- or 6-membered ring heteroaromatic, preferably 3-pyridinyl and 5-pyrimidinyl moieties, and the alkyl group is typically a C1-4 alkyl. The substituent at the 3-position of the 1-azabicycloalkane is a carbonyl group-containing moiety, such as an amide, carbamate, urea, thioamide, thiocarbamate, thiourea or similar functionality. The compounds exhibit activity at nicotinic acetylcholine receptors (nAChRs), particularly the α7 nAChR subtype, and are useful towards modulating neurotransmission and the release of ligands involved in neurotransmission. Methods for preventing or treating conditions and disorders, including central nervous system (CNS) disorders, which are characterized by an alteration in normal neurotransmission, are also disclosed. Also disclosed are methods for treating inflammation, autoimmune disorders, pain and excess neovascularization, such as that associated with tumor growth.
Owner:ATTENUA INC
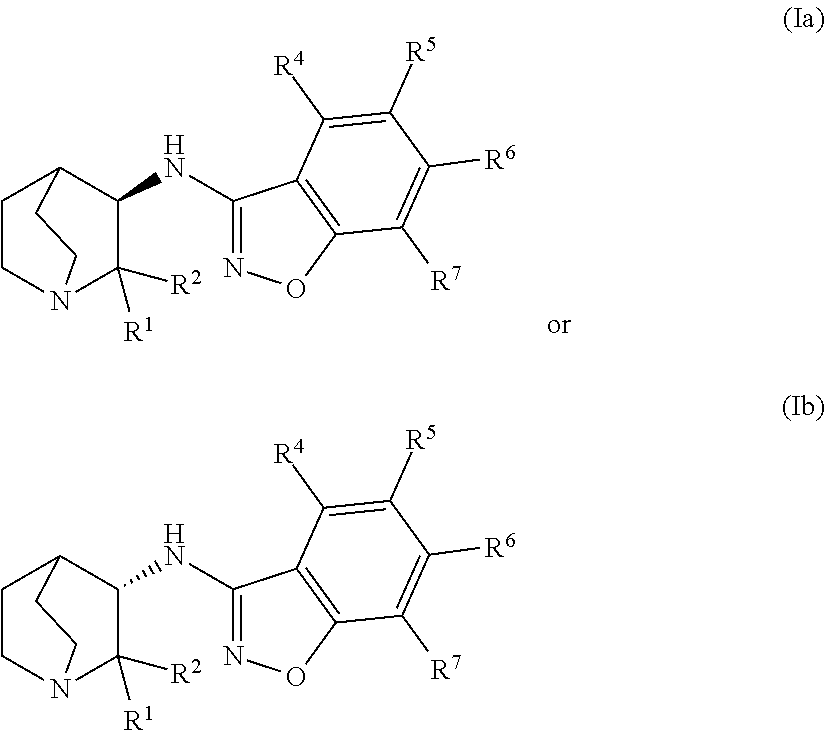
Fused bicycloheterocycle substituted quinuclidine derivatives
InactiveUS20050245531A1Improve cognitive functionLimited abilityBiocideNervous disorderQuinuclidineΑ7 nachr
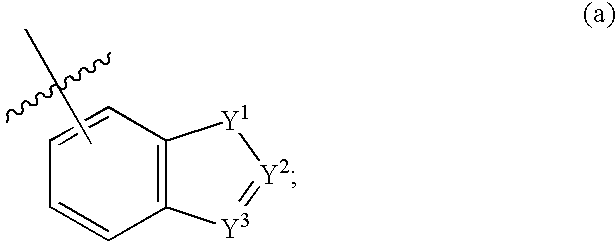
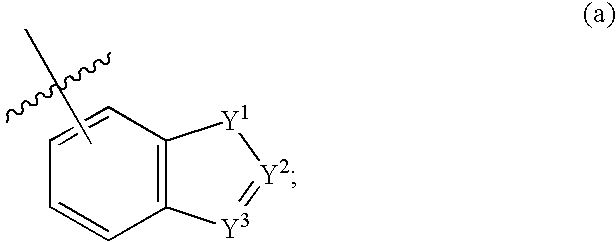
Compounds of formula (I) wherein n is 0, 1, or 2; A is N or N+—O—; X is O, S, —NH—, and —N-alkyl-; Ar1 is a 6-membered aromatic ring; and Ar2 is a fused bicycloheterocycle. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:ABBOTT LAB INC
Fused bicycloheterocycle substituted quinuclidine derivatives
InactiveUS20050137204A1Improve cognitive functionLimited abilityBiocideOrganic chemistryQuinuclidineΑ7 nachr
Compounds of formula (I) wherein n is 0, 1, or 2; A is N or N+—O−; X is O, S, —NH—, and —N-alkyl-; Ar1 is a 6-membered aromatic ring; and Ar2 is a fused bicycloheterocycle. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:ABBOTT LAB INC
Treatment of diseases with combinations of alpha 7 Nicotinic Acetylcholine Receptor agonists and other compounds
The present invention relates to compositions and methods to treat diseases or condition with an α7 nAChR full agonist and an inhibitor of cholinesterase, and or beta secretase and or gamma secretase.
Owner:CORBETT JEFFREY W +1
Fused bicycloheterocycle substituted quinuclidine derivatives
ActiveUS7160876B2Improve cognitive functionLimited abilityBiocideNervous disorderQuinuclidineΑ7 nachr
Compounds of formula (I)wherein n is 0, 1, or 2; X is O, S, —NH—, and —N-alkyl-; Ar1 is a 6-membered aromatic ring; and Ar2 is a fused bicycloheterocycle. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:ABBVIE INC
Fused bicycloheterocycle substituted quinuclidine derivatives
ActiveUS20050137184A1Improve cognitive functionLimited abilityBiocideNervous disorderQuinuclidineΑ7 nachr
Compounds of formula (I) wherein n is 0, 1, or 2; X is O, S, —NH—, and —N-alkyl-; Ar1 is a 6-membered aromatic ring; and Ar2 is a fused bicycloheterocycle. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:ABBVIE INC
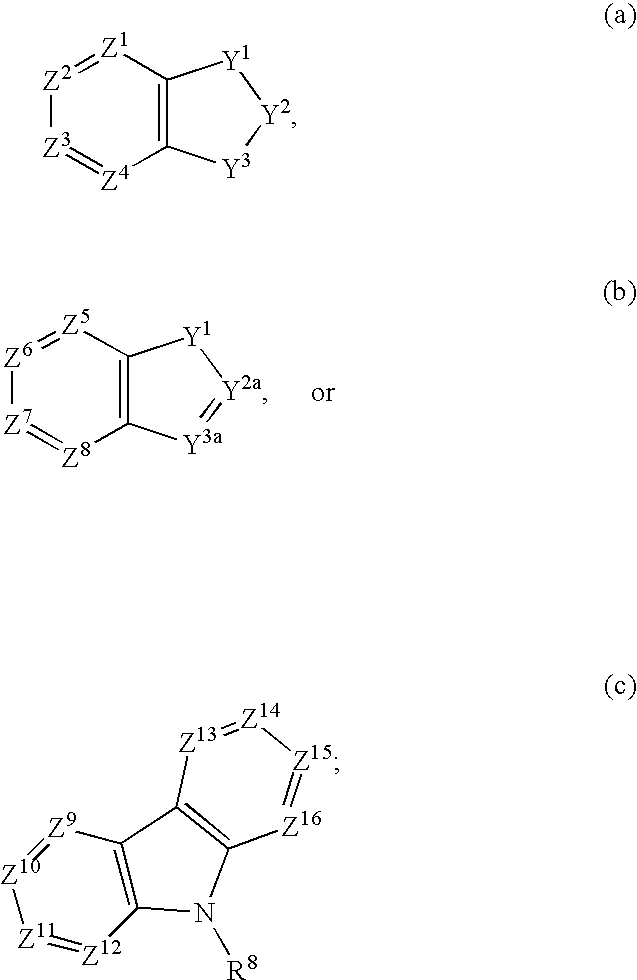
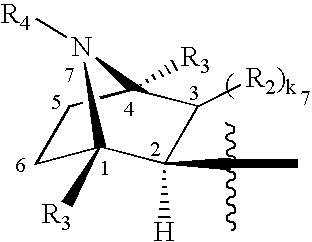
3-Quinuclidinyl amino-substituted biaryl derivatives
InactiveUS20050159597A1Improve cognitive functionLimited abilityBiocideNervous disorderStereochemistryΑ7 nachr
Compounds of formula (I) wherein A is N or N+—O−; n is 0, 1, or 2; Y is O, S, —NH—, and —N-alkyl-; Ar1 is both 6-membered aromatic rings; Ar2 is 5- or 6-membered aromatic rings with a —NR8R9 group, as defined herein. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:ABBVIE INC
3-quinuclidinyl amino-substituted biaryl derivatives
InactiveUS20050137203A1Improve cognitive functionLimited abilityBiocideNervous disorderStereochemistryΑ7 nachr
Compounds of formula (I) wherein n is 0, 1, or 2; Y is O, S, —NH—, and —N-alkyl-; Ar1 is both 6-membered aromatic rings; Ar2 is 5- or 6-membered aromatic rings with a —NR8R9 group, as defined herein. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:ABBOTT LAB INC
Positive allosteric modulators of the nicotinic acetylcholine receptor
InactiveUS20050250816A1High activityIncrease ACh levelAntibacterial agentsBiocideDiseaseAllosteric modulator
Owner:PFIZER INC
3-Substituted-2(arylalkyl)-1-azabicycloalkanes and methods of use thereof
InactiveUS20050255040A1Useful towards modulating release of ligandsWithout appreciable side effectAntibacterial agentsNervous disorderThiocarbamateDisease
The present invention relates to 3-substituted-2-(arylalkyl)-1-azabicycloalkanes, methods of preparing the compounds and methods of treatment using the compounds. The azabicycloalkanes generally are azabicycloheptanes, azabicyclooctanes, or azabicyclononanes. The aryl group in the arylalkyl moiety is a 5- or 6-membered ring heteroaromatic, preferably 3-pyridinyl and 5-pyrimidinyl moieties, and the alkyl group is typically a C1-4 alkyl. The substituent at the 3-position of the 1 -azabicycloalkane is a carbonyl group-containing moiety, such as an amide, carbamate, urea, thioamide, thiocarbamate, thiourea or similar functionality. The compounds exhibit activity at nicotinic acetylcholine receptors (nAChRs), particularly the α7 nAChR subtype, and are useful towards modulating neurotransmission and the release of ligands involved in neurotransmission. Methods for preventing or treating conditions and disorders, including central nervous system (CNS) disorders, which are characterized by an alteration in normal neurotransmission, are also disclosed. Also disclosed are methods for treating inflammation, autoimmune disorders, pain and excess neovascularization, such as that associated with tumor growth.
Owner:ATTENUA INC
3-Substituted-2(Arylalkyl)-1-Azabicycloalkanes and Methods of Use Thereof
InactiveUS20060247270A1Useful towards modulating release of ligandsWithout appreciable side effectAntibacterial agentsBiocideDiseaseThiocarbamate
The present invention relates to 3-substituted-2-(arylalkyl)-1-azabicycloalkanes, methods of preparing the compounds and methods of treatment using the compounds. The azabicycloalkanes generally are azabicycloheptanes, azabicyclooctanes, or azabicyclononanes. The aryl group in the arylalkyl moiety is a 5- or 6-membered ring heteroaromatic, preferably 3-pyridinyl and 5-pyrimidinyl moieties, and the alkyl group is typically a C1-4 alkyl. The substituent at the 3-position of the 1-azabicycloalkane is a carbonyl group-containing moiety, such as an amide, carbamate, urea, thioamide, thiocarbamate, thiourea or similar functionality. The compounds exhibit activity at nicotinic acetylcholine receptors (nAChRs), particularly the α7 nAChR subtype, and are useful towards modulating neurotransmission and the release of ligands involved in neurotransmission. Methods for preventing or treating conditions and disorders, including central nervous system (CNS) disorders, which are characterized by an alteration in normal neurotransmission, are also disclosed. Also disclosed are methods for treating inflammation, autoimmune disorders, pain and excess neovascularization, such as that associated with tumor growth.
Owner:ATTENUA INC
Positive allosteric modulators of the nicotinic acetylcholine receptor
InactiveUS20080132551A1High activityEnhance the activity of the α7 nAChRBiocideSenses disorderDiseaseAllosteric modulator
Owner:PFIZER INC
Postsynaptically targeted chemodenervation agents and their methods of use
ActiveUS9815875B2Low toxicityDuration rangeNervous disorderPeptide/protein ingredientsChemodenervationΑ7 nachr
Owner:MYOCEPT
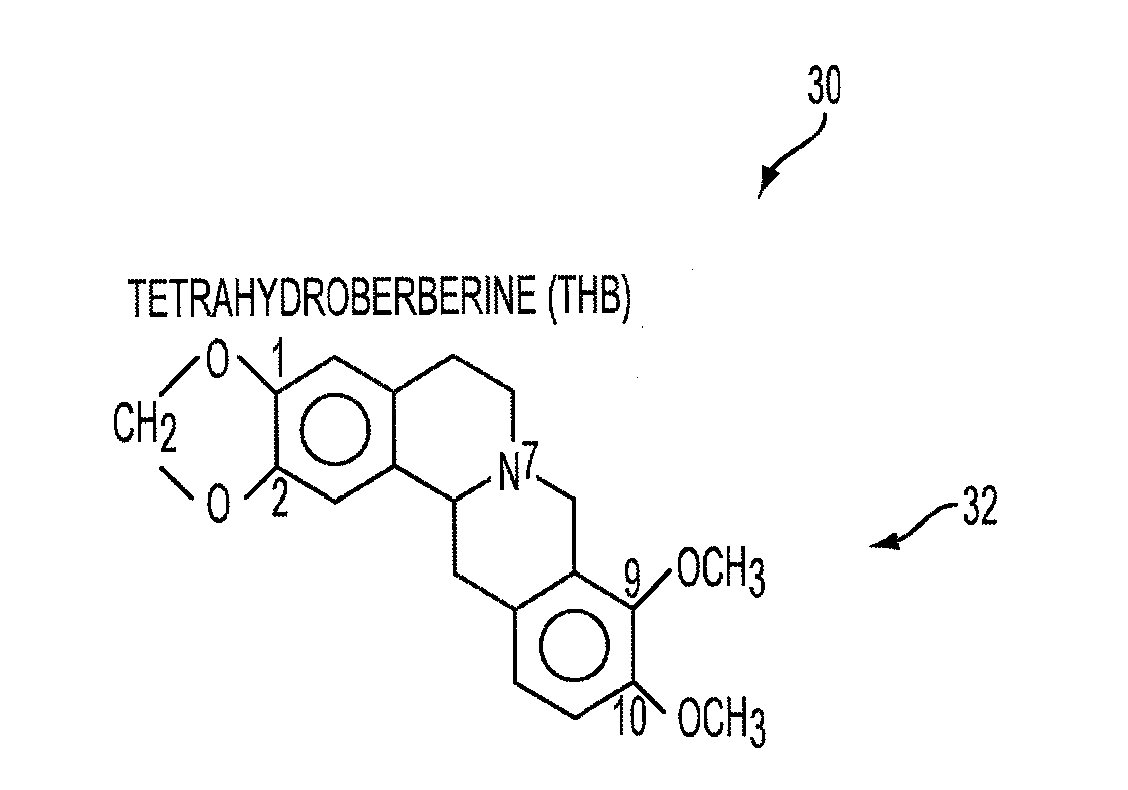
Method for Decreasing Nicotine and Other Substance Use in Humans
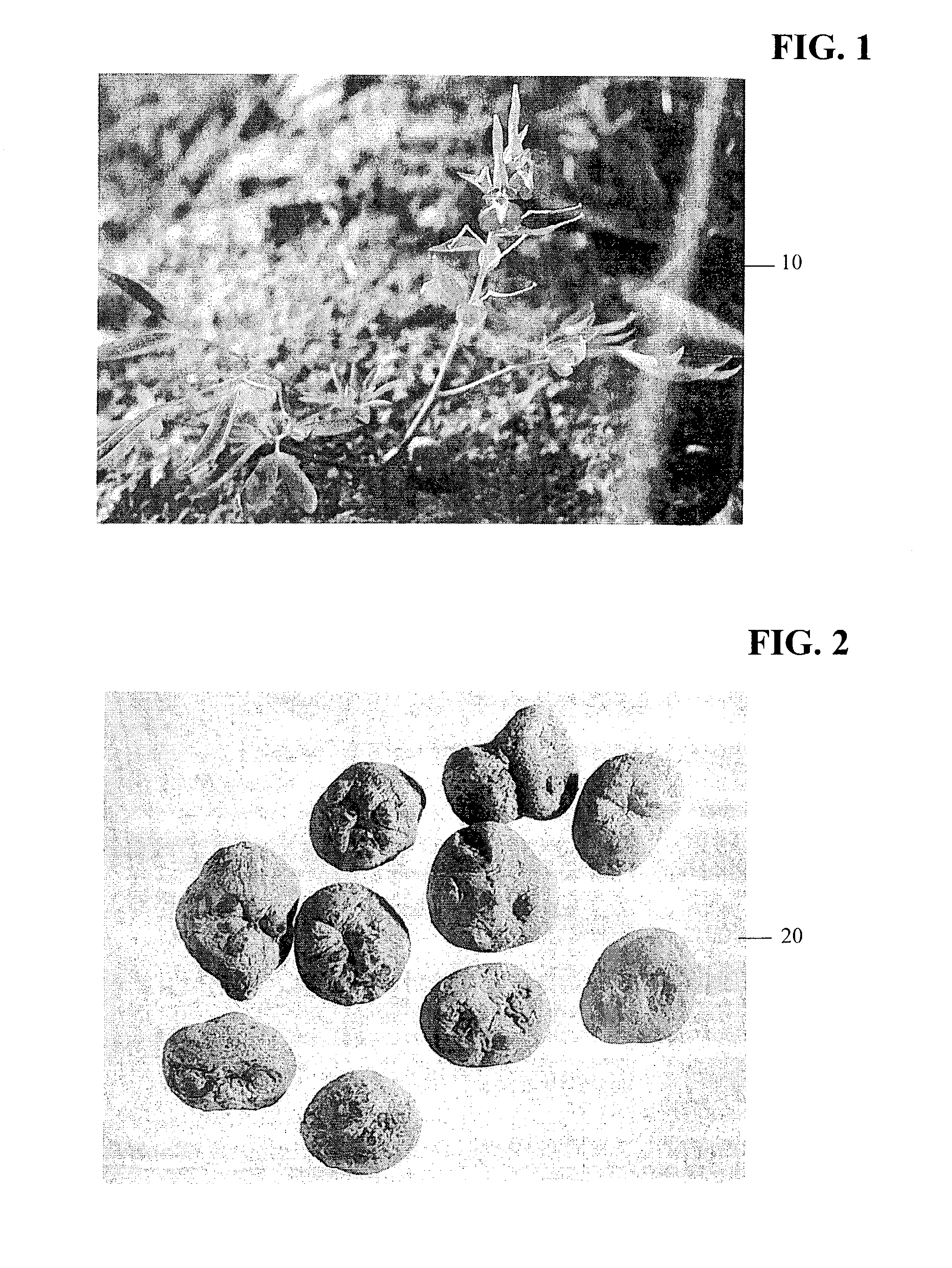
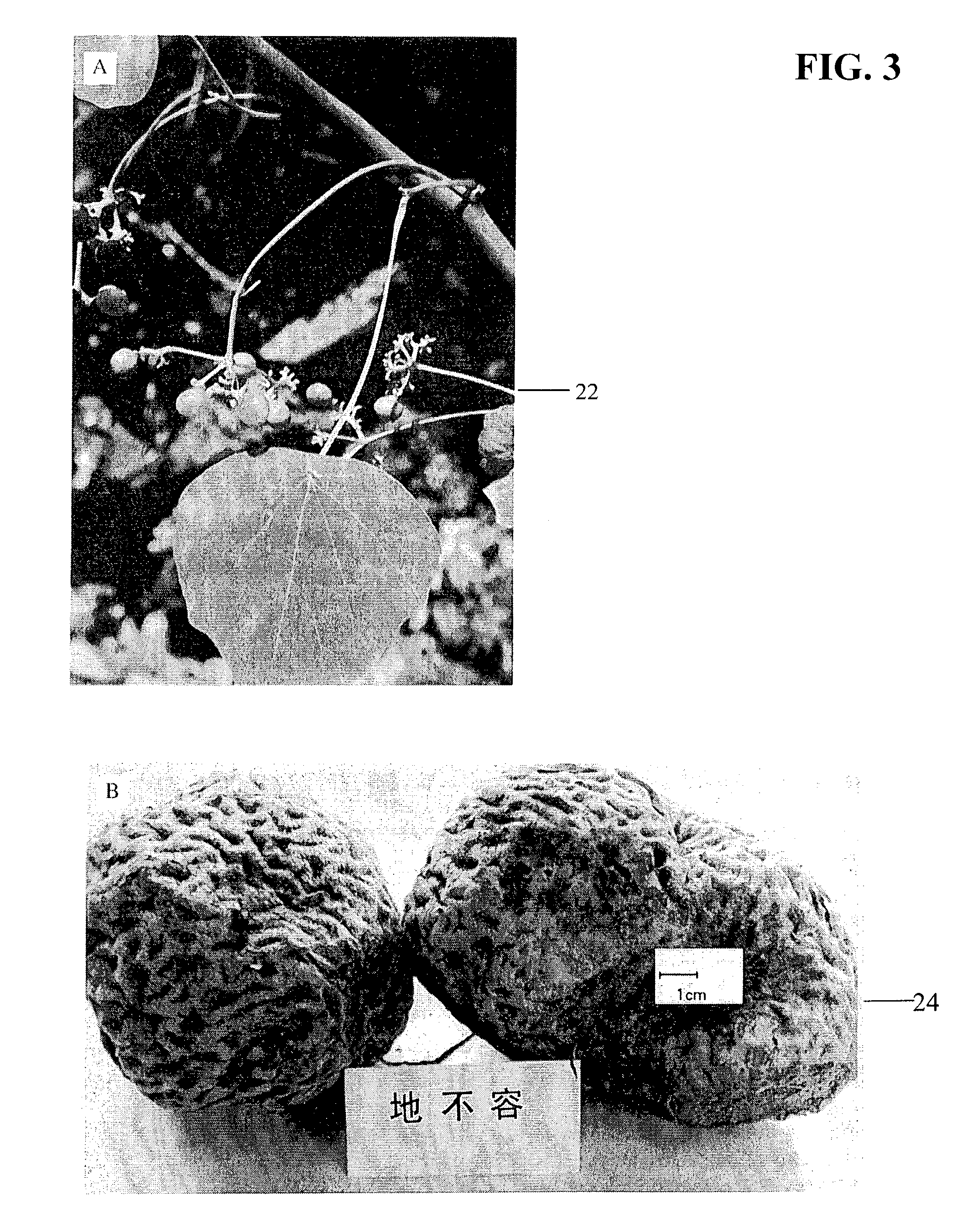
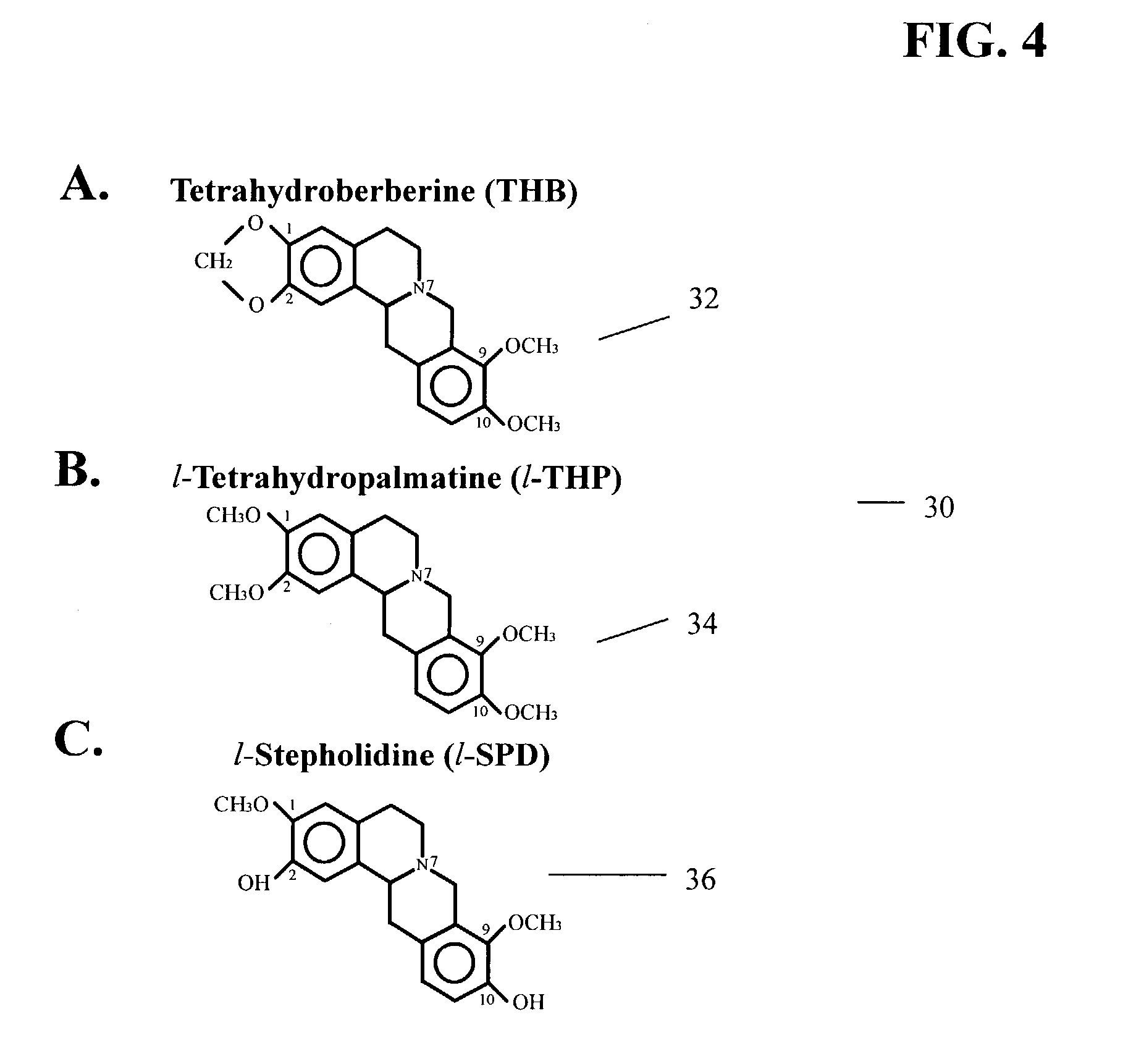
A method for decreasing nicotine and other substance use in humans is disclosed. Tetrahydroberberine (THB) and its analogs, l-Tetrahydropalmatine (l-THP) and l-Stepholidine (l-SPD), are present in and can be isolated from several plants in the Magnoliidae superorder. According to the disclosed method, THB and its analogs are used to block nicotine-induced DA release, and modulate heterologous or homoeric expression of human nicotinic acetylcholine receptors (nAChR) in humans. Specifically, THB exhibits bi-directory modulation of α4β2-nAChR-mediated currents induced by nicotine. THB also shows predominant inhibition on homologously expressed α7-nAChR function. Thus, according to the disclosed method, THB is used to simultaneous blockade midbrain DA system function, the brain reward center, and neuronal α4β2- and α7-nAChR function, the major nicotine targets in the brain. Therefore, THB and its analogs serve as a novel class of natural compounds to decrease nicotine dependence in humans. Furthermore other substances, such as alcohol, cocaine, and opiates, also operate by triggering the brain reward center, resulting in a cycle of substance or alcohol abuse. THB and its analogs can be used to decrease use of substances such as alcohol, cocaine, and opiates. Finally, because THB and its analogs are DA antagonists, THB and its analogs can also be used as a treatment for Parkinson's Disease, Alzheimer's Disease and Schizophrenia.
Owner:ARIZONA HEALTH CONSULTING GROUP
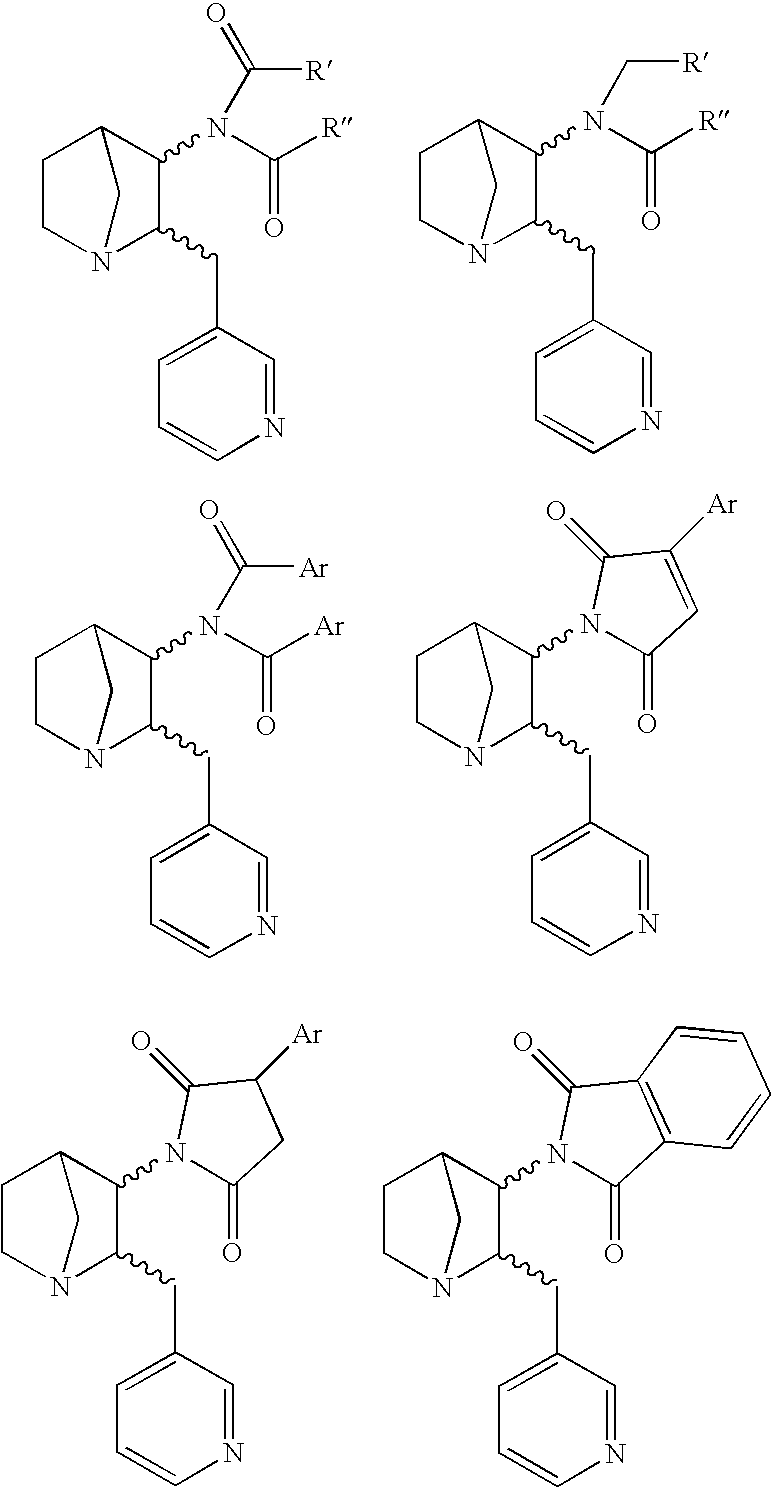
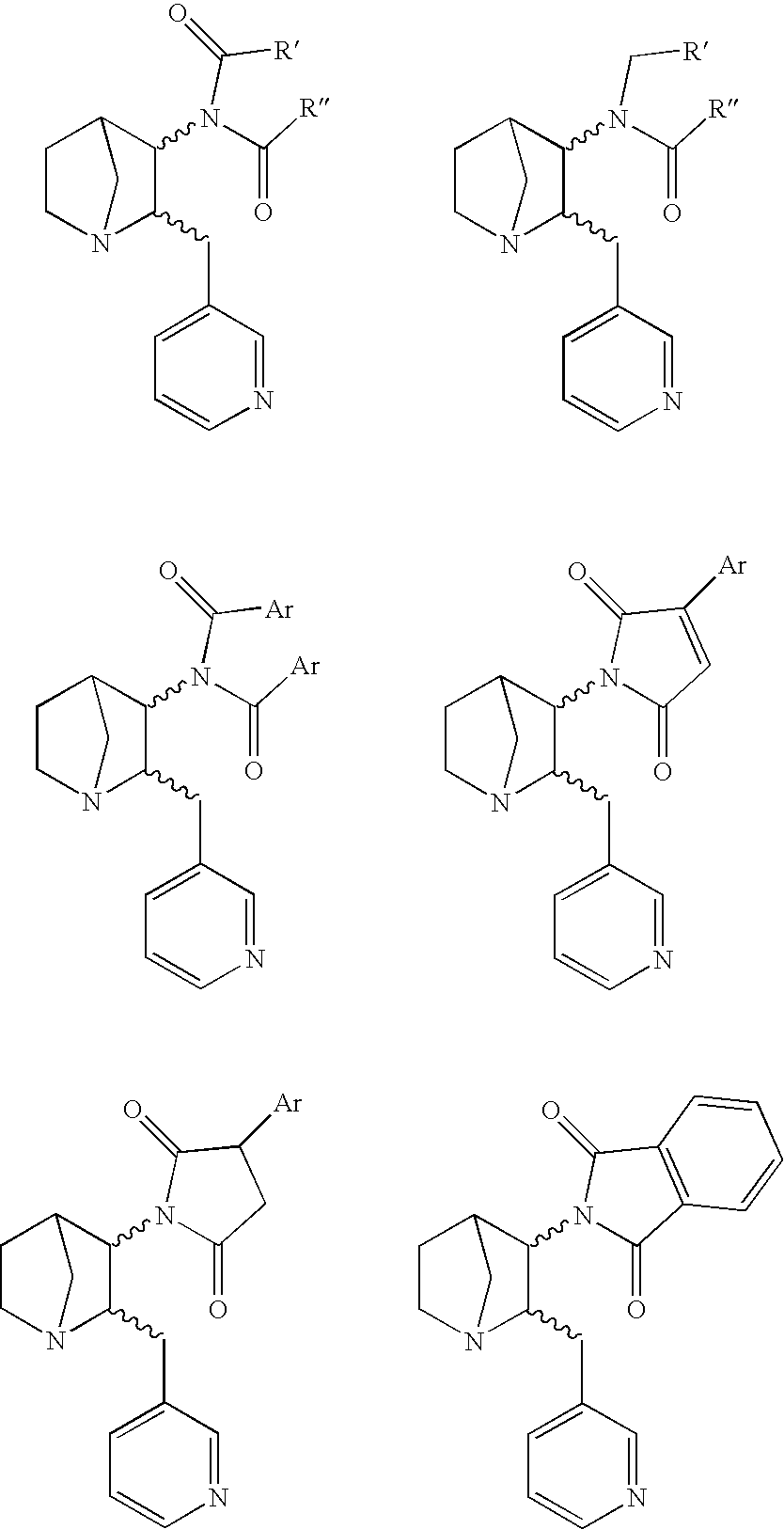
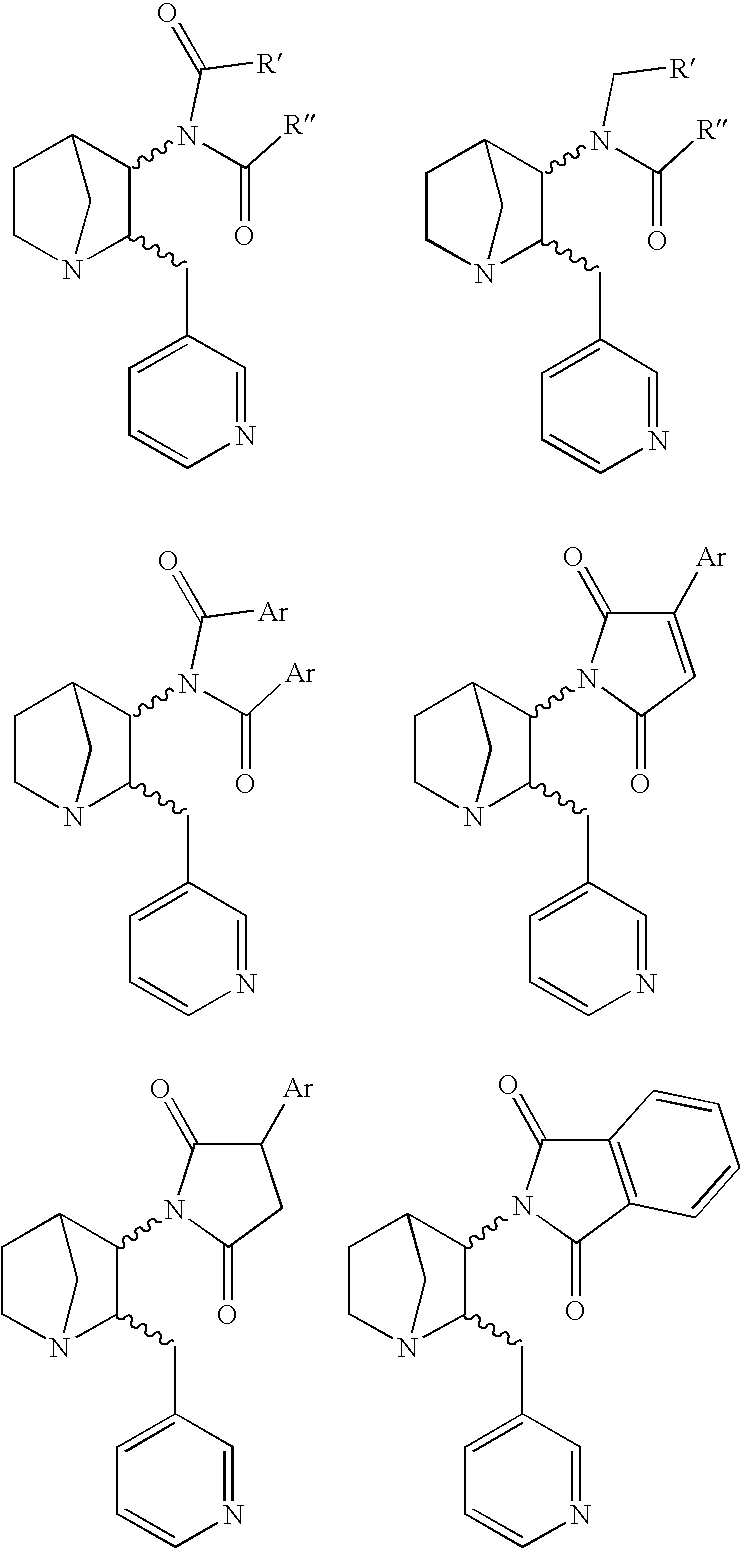
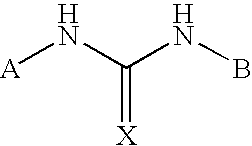
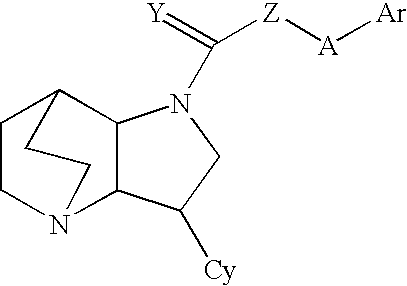
Heteroaryl-Substituted Diazatricycloalkanes and Methods of Use Thereof
InactiveUS20070197579A1Modulate activityWithout side effectAntibacterial agentsBiocideUrea derivativesAlkane
The present invention relates to amide and urea derivatives of heteroaryl-substituted diazatricycloalkanes, pharmaceutical compositions including the compounds, methods of preparing the compounds, and methods of treatment using the compounds. More specifically, the methods of treatment involve modulating the activity of the α7 nAChR subtype by administering one or more of the compounds to treat or prevent disorders mediated by the α7 nAChR subtype. The diazatricycloalkanes typically consist of a 1-azabicyclooctane fused to pyrrolidine ring. The substitutent heteroaryl groups are 5- or 6-membered ring heteroaromatics, such as 3-pyridinyl and 5-pyrimidinyl moieties, which are attached directly to the diazatricycloalkane. The secondary nitrogen of the pyrrolidine moiety is substituted with an arylcarbonyl (amide type derivative) or an arylaminocarbonyl (N-arylcarbamoyl) (urea type derivative) group. The compounds are beneficial in therapeutic applications requiring a selective interaction at certain nAChR subtypes. That is, the compounds modulate the activity of certain nAChR subtypes, particularly the α7 nAChR subtype, and do not have appreciable activity toward muscarinic receptors. Radiolabeled versions of the compounds can be used in diagnostic methods.
Owner:TARGACEPT INC
Treatment of attention defecit hyperactivity disorder
InactiveUS20050107425A1Highly effectiveEffective treatmentBiocideNervous disorderStimulantDepressant
Owner:PFIZER INC
Heteroaryl-substituted diazatricycloalkanes and methods of use thereof
InactiveUS7732607B2Useful towards modulating release of ligandsWithout appreciable side effectAntibacterial agentsBiocideUrea derivativesAryl
The present invention relates to amide and urea derivatives of heteroaryl-substituted diazatricycloalkanes, pharmaceutical compositions including the compounds, methods of preparing the compounds, and methods of treatment using the compounds. More specifically, the methods of treatment involve modulating the activity of the α7 nAChR subtype by administering one or more of the compounds to treat or prevent disorders mediated by the α7 nAChR subtype. The diazatricycloalkanes typically consist of a 1-azabicyclooctane fused to pyrrolidine ring. The substitutent heteroaryl groups are 5- or 6-membered ring heteroaromatics, such as 3-pyridinyl and 5-pyrimidinyl moieties, which are attached directly to the diazatricycloalkane. The secondary nitrogen of the pyrrolidine moiety is substituted with an arylcarbonyl (amide type derivative) or an arylaminocarbonyl (N-arylcarbamoyl) (urea type derivative) group. The compounds are beneficial in therapeutic applications requiring a selective interaction at certain nAChR subtypes. That is, the compounds modulate the activity of certain nAChR subtypes, particularly the α7 nAChR subtype, and do not have appreciable activity toward muscarinic receptors. Radiolabeled versions of the compounds can be used in diagnostic methods.
Owner:TARGACEPT INC
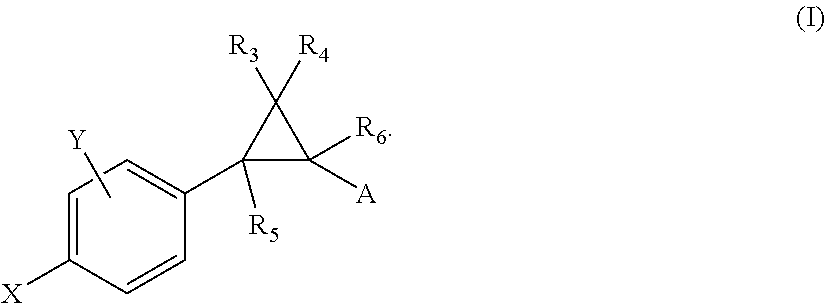
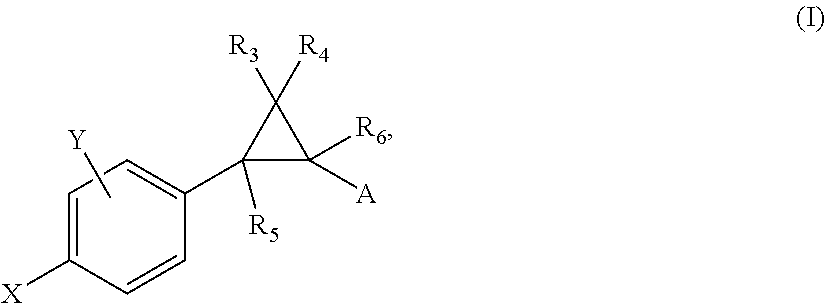
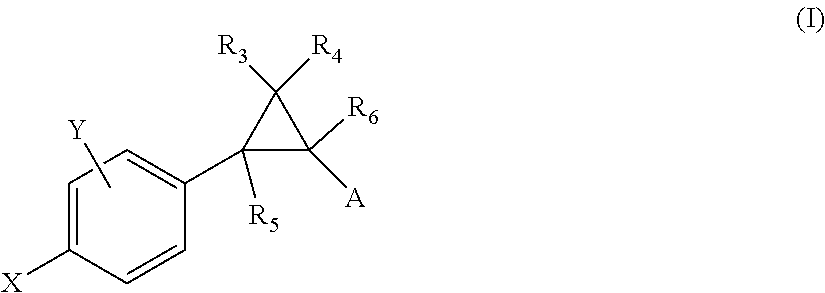
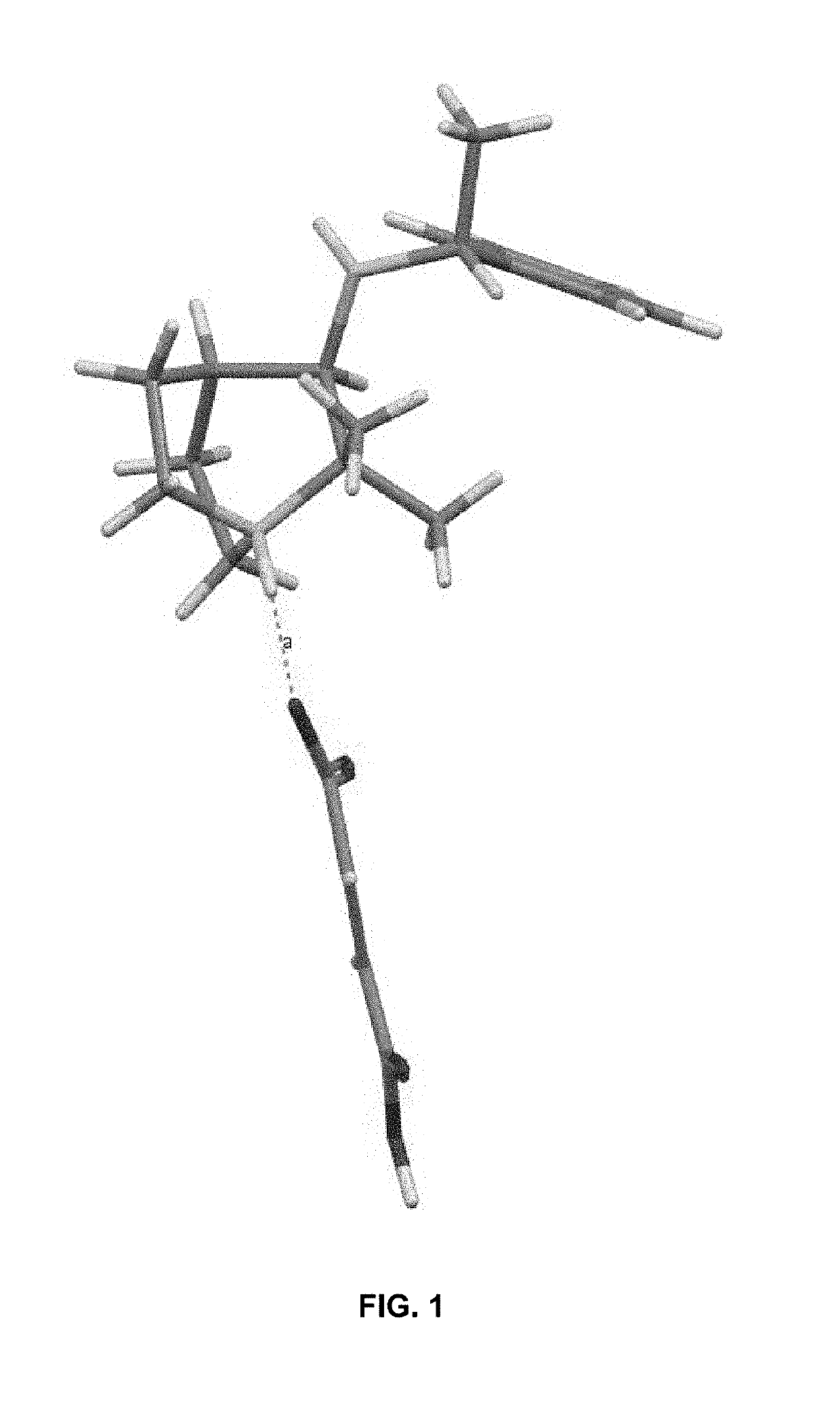
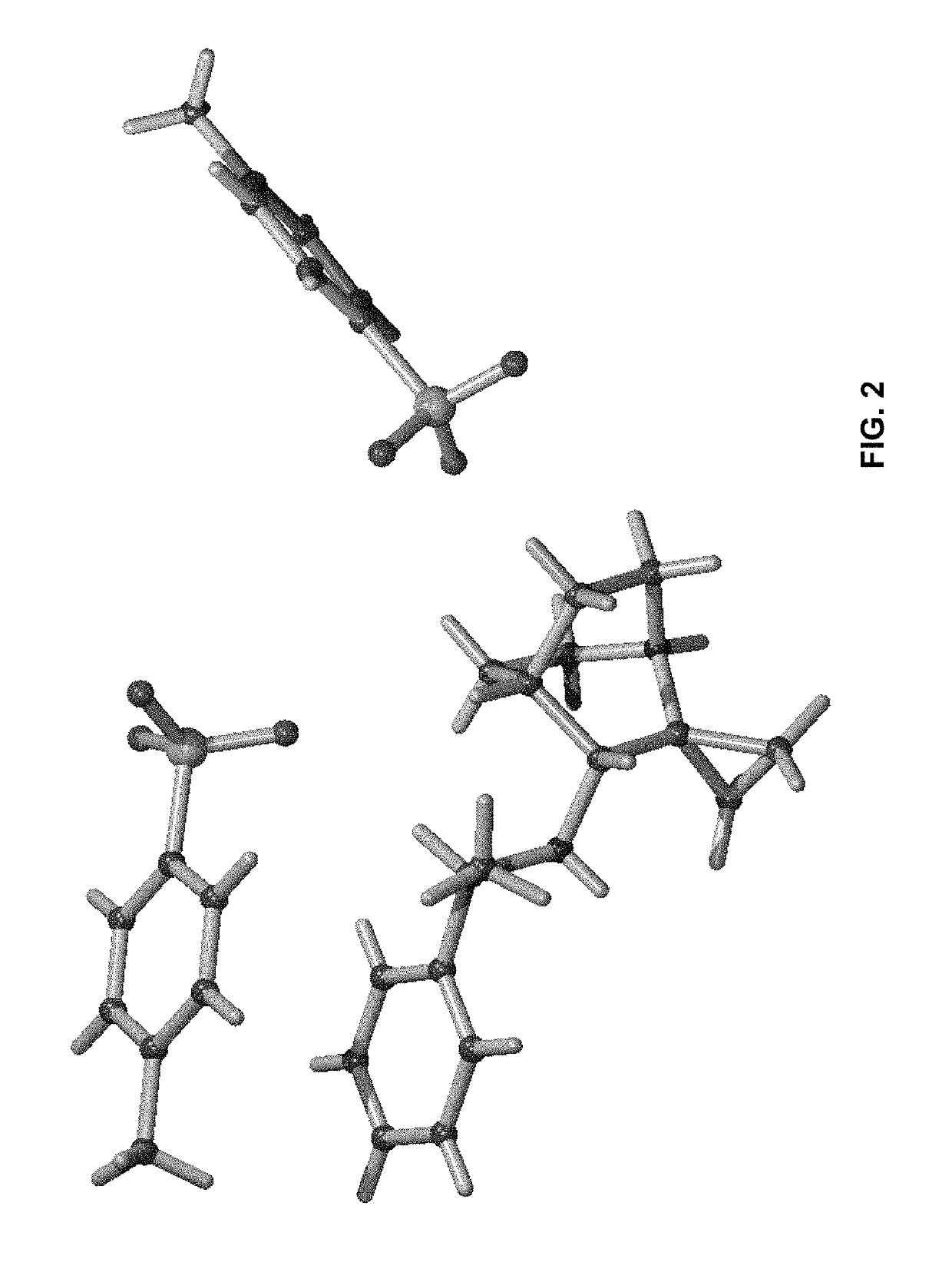
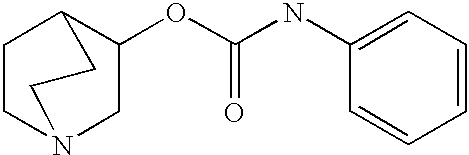
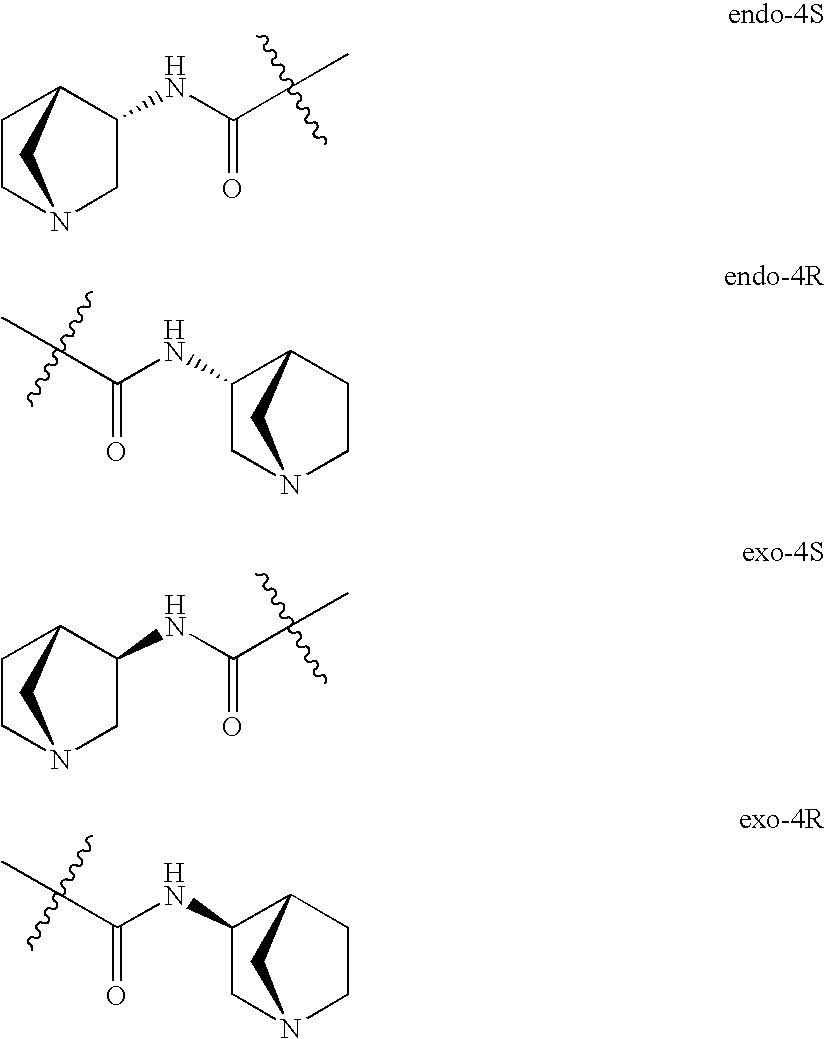
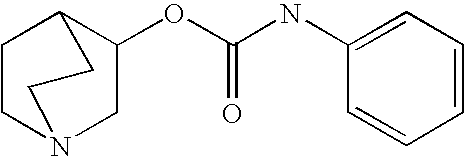
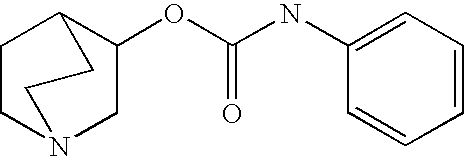
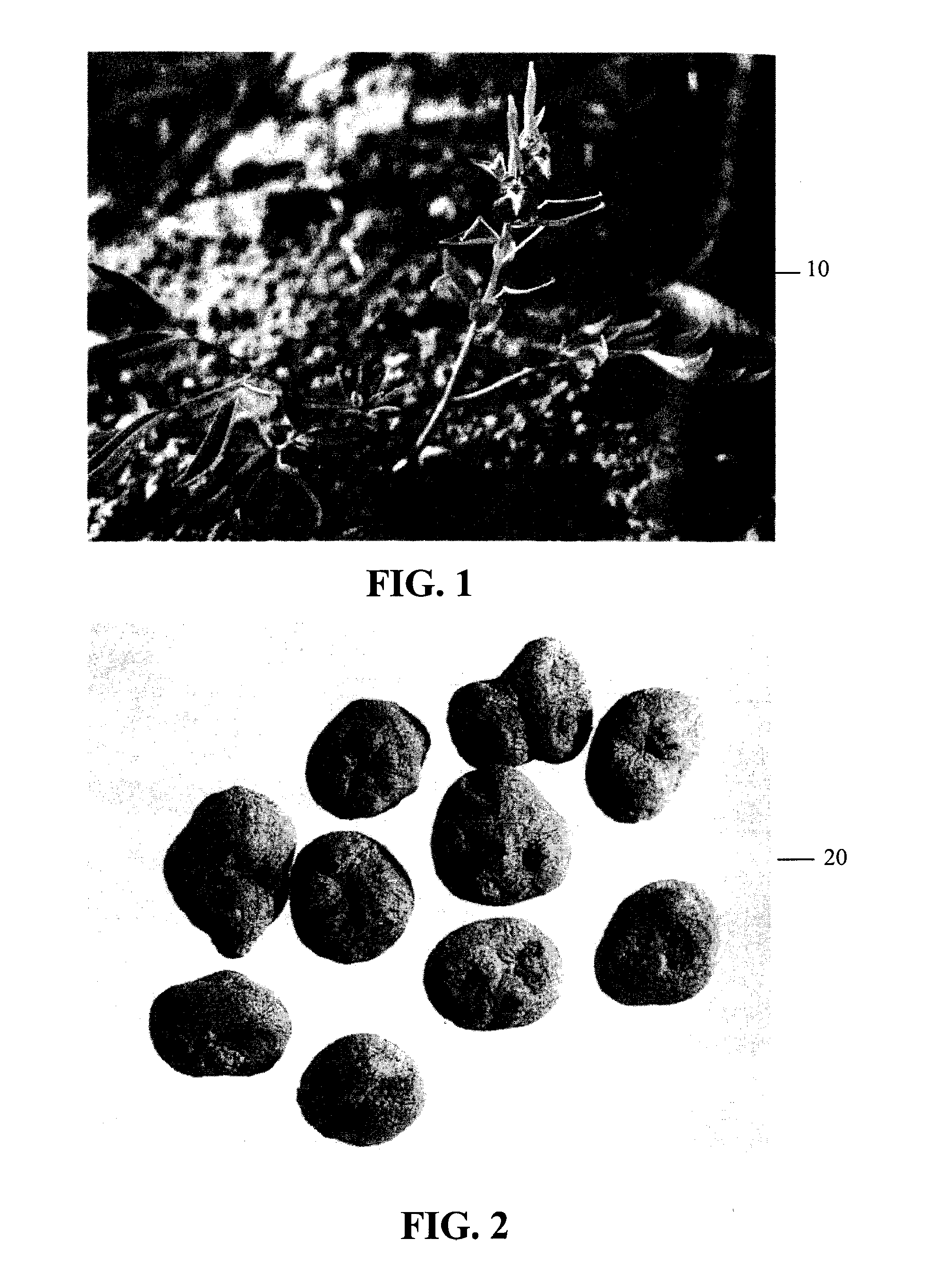
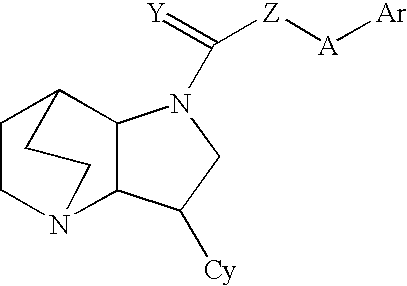
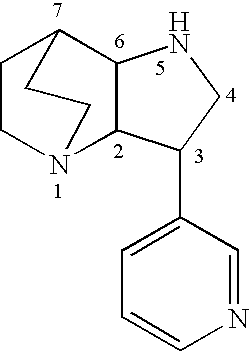
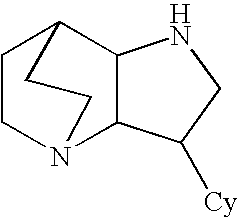
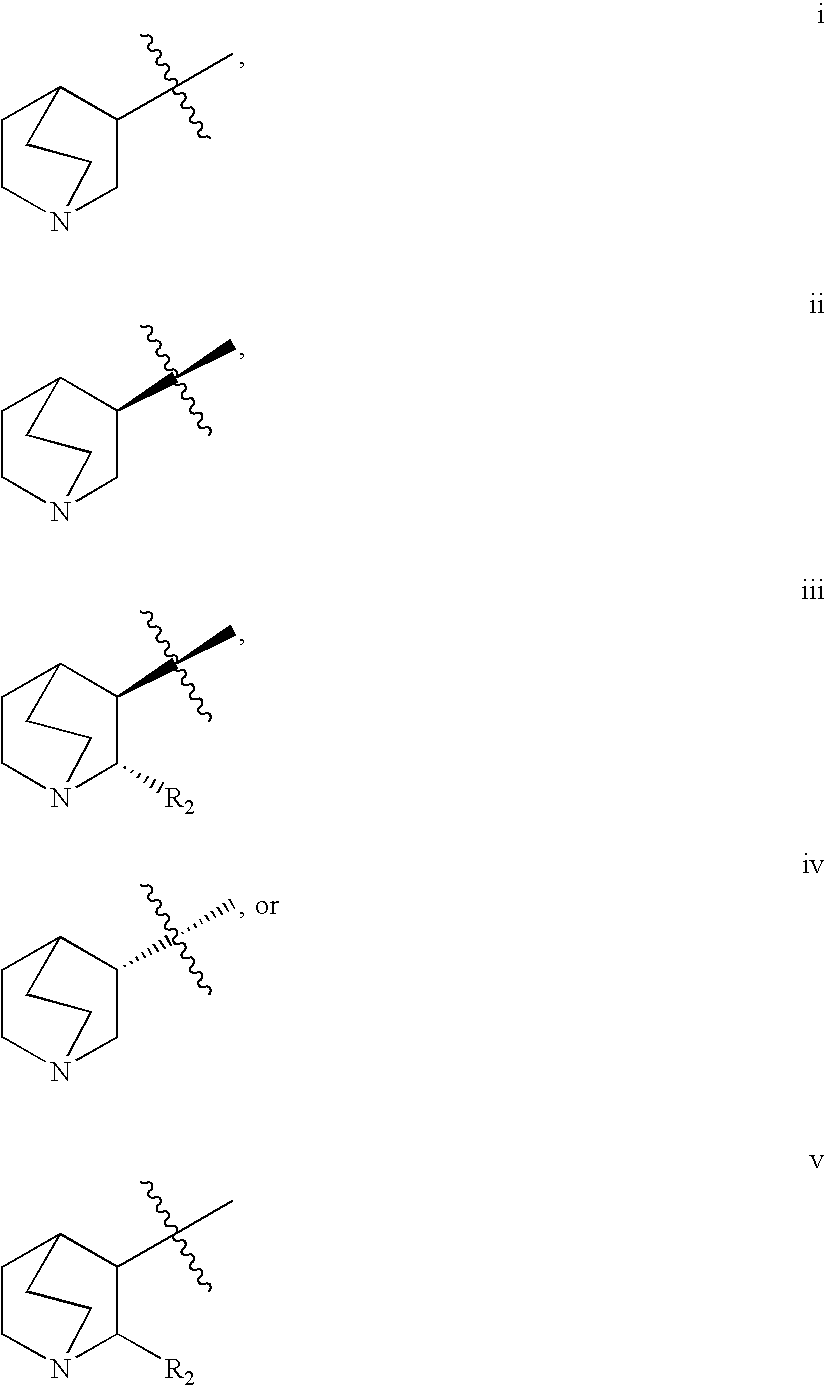
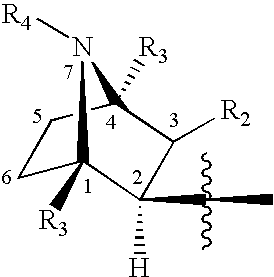
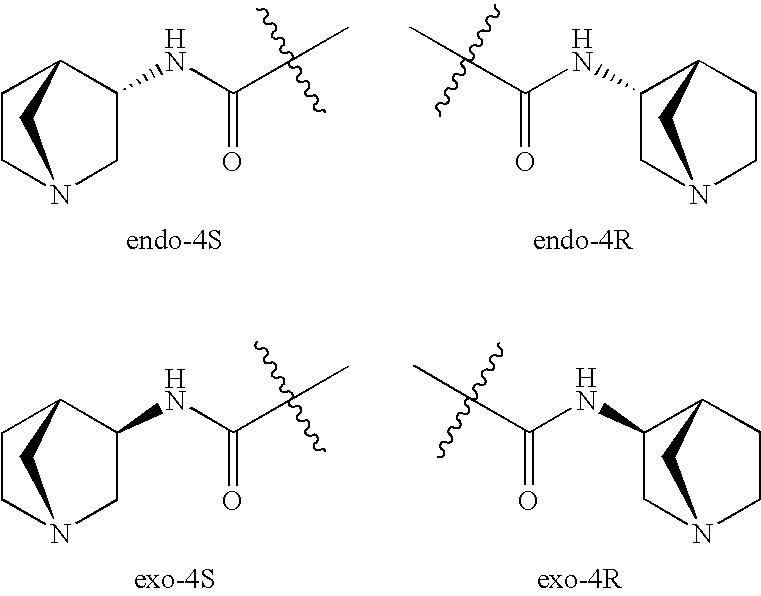
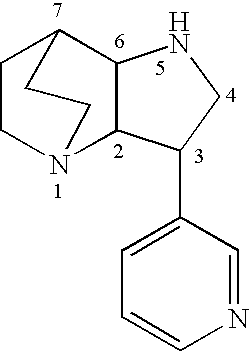
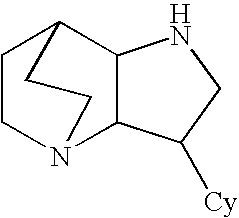
Crystalline fumarate salts of 1-azabicyclo[2.2.2]oct substituted furo[2,3-c]pyridinyl carboxamide and compositions and preparations thereof
The invention provides fumarate salts of N-[1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide, compositions, racemic mixtures, or pure enantiomers thereof, and preparation thereof. The fumarate salts are useful to treat diseases or conditions in which α7 nAChR is known to be involved.
Owner:PFIZER INC
Method for decreasing nicotine and other substance use in humans
A method for decreasing nicotine and other substance use in humans is disclosed. Tetrahydroberberine (THB) and its analogs, l-Tetrahydropalmatine (l-THP) and l-Stepholidine (l-SPD), are present in and can be isolated from several plants in the Magnoliidae superorder. According to the disclosed method, THB and its analogs are used to block nicotine-induced DA release, and modulate heterologous or homoeric expression of human nicotinic acetylcholine receptors (nAChR) in humans. Specifically, THB exhibits bi-directory modulation of α4β2-nAChR-mediated currents induced by nicotine. THB also shows predominant inhibition on homologously expressed α7-nAChR function. Thus, according to the disclosed method, THB is used to simultaneous blockade midbrain DA system function, the brain reward center, and neuronal α4β2- and α7-nAChR function, the major nicotine targets in the brain. Therefore, THB and its analogs serve as a novel class of natural compounds to decrease nicotine dependence in humans. Furthermore other substances, such as alcohol, cocaine, and opiates, also operate by triggering the brain reward center, resulting in a cycle of substance or alcohol abuse. THB and its analogs can be used to decrease use of substances such as alcohol, cocaine, and opiates. Finally, because THB and its analogs are DA antagonists, THB and its analogs can also be used as a treatment for Parkinson's Disease, Alzheimer's Disease and Schizophrenia.
Owner:WU JIE
Antitussive compositions and methods
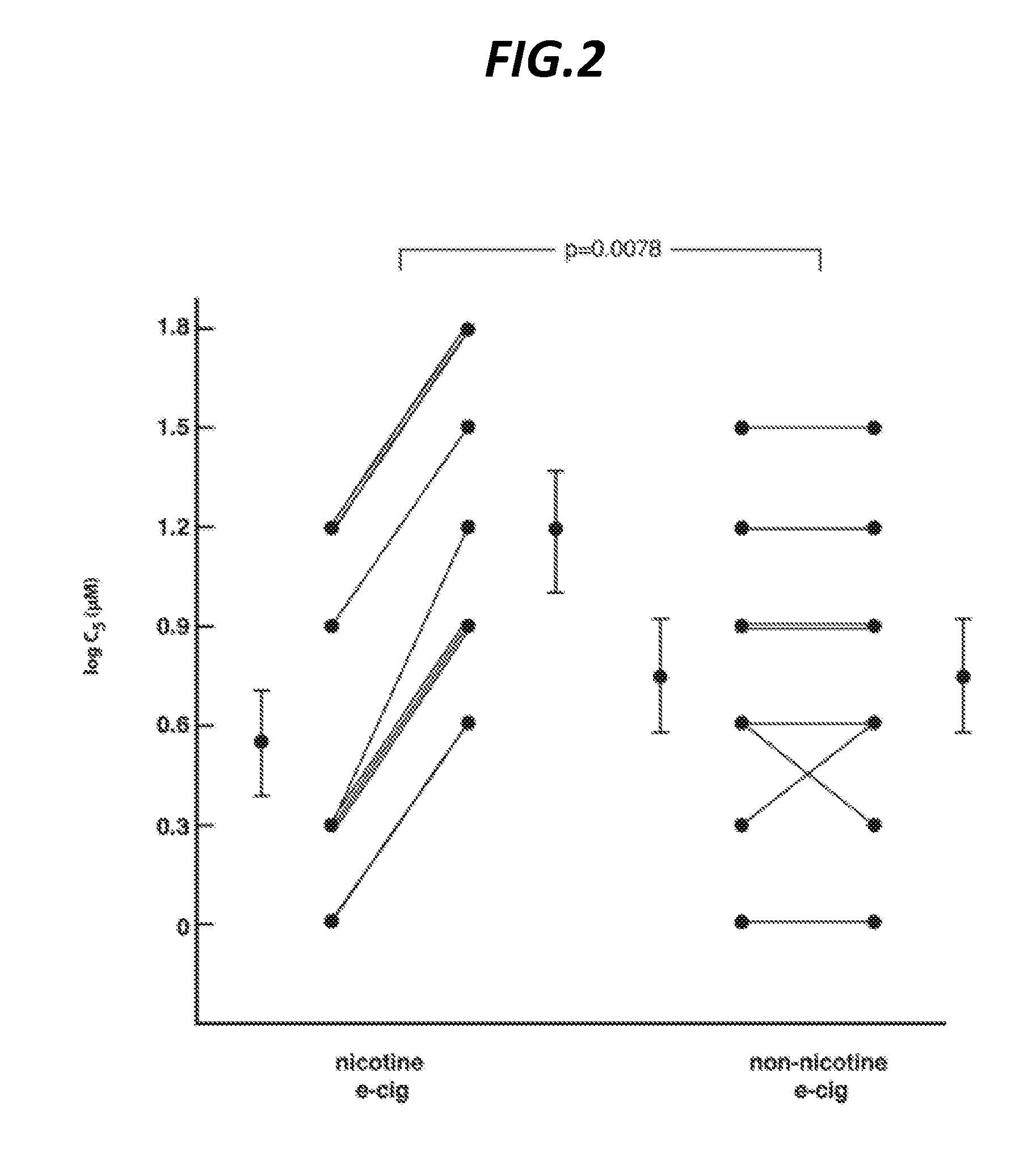
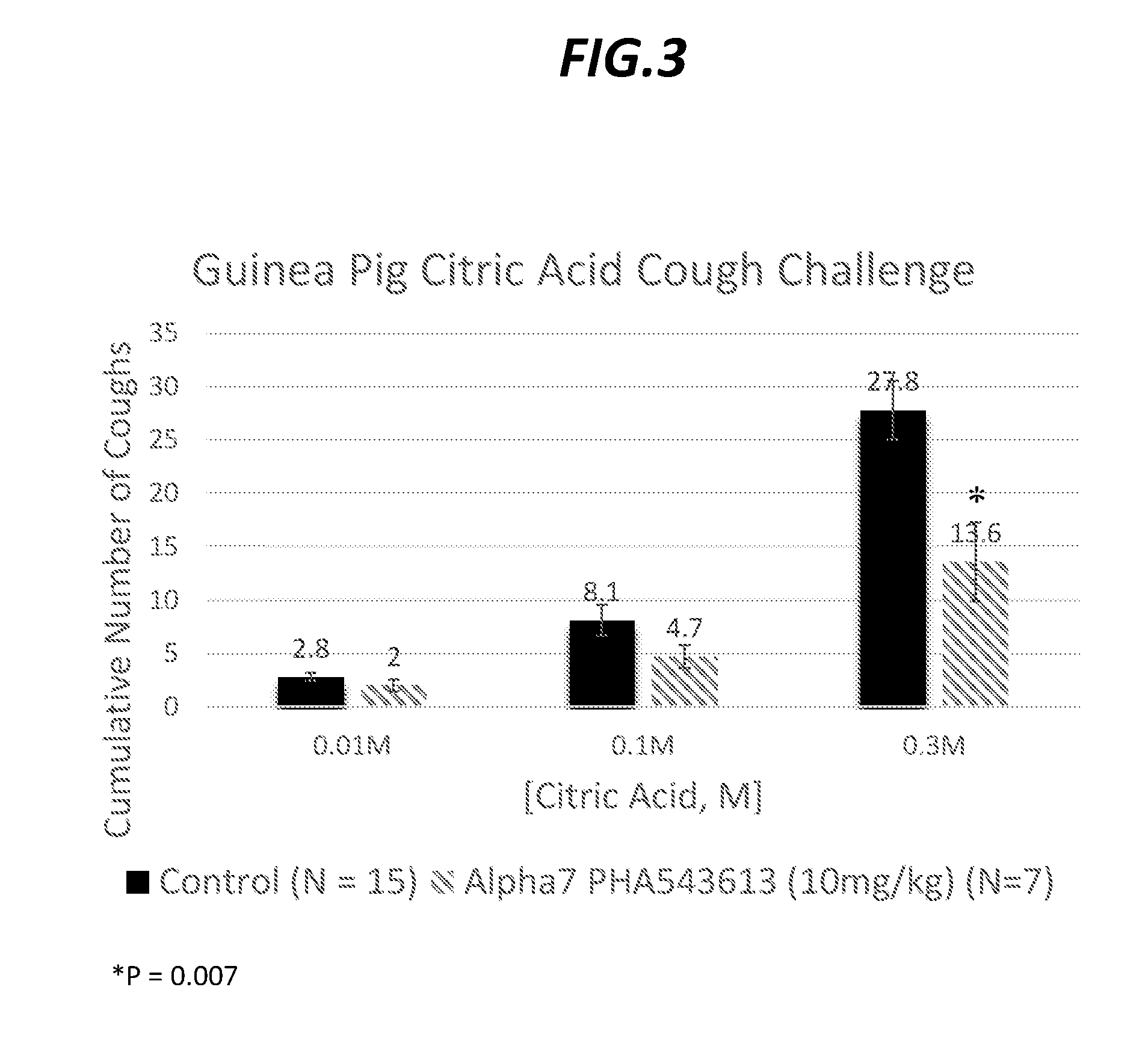
InactiveUS20170027918A1Suppressing coughPowder deliverySpray deliveryNicotinic Receptor AgonistStereochemistry
Disclosed herein are compositions which include nicotinic receptor agonists, specifically of the α7 nAChR subtype, and methods for suppressing cough.
Owner:ATTENUA INC
Allosteric modulators of nicotinic acetylcholine receptors
The present disclosure relates to compounds of formula I that are useful as modulators of α7 nAChR, compositions comprising such compounds, and the use of such compounds for preventing, treating, or ameliorating disease, particularly disorders of the central nervous system such as cognitive impairments in Alzheimer's disease, Parkinson's disease, and schizophrenia, as well as for L-DOPA induced-dyskinesia and inflammation
Owner:MERCK SHARP & DOHME LLC
Allosteric modulators of nicotinic acetylcholine receptors
The present disclosure relates to compounds of formula I that are useful as modulators of α7 nAChR, compositions comprising such compounds, and the use of such compounds for preventing, treating, or ameliorating disease, particularly disorders of the central nervous system such as cognitive impairments in Alzheimer's disease, Parkinson's disease, and schizophrenia, as well as for L-DOPA induced-dyskinesia and inflammation
Owner:MERCK SHARP & DOHME LLC
Postsynaptically Targeted Chemodenervation Agents and Their Methods of Use
ActiveUS20110118190A1Low toxicityDuration rangeNervous disorderPeptide/protein ingredientsChemodenervationΑ7 nachr
Owner:MYOCEPT
Geminal substituted quinuclidine amide compounds as agonists of α-7 nicotonic acetylcholine receptors
InactiveUS10183938B2Increase awarenessPositive effect on clinical functionOrganic active ingredientsNervous disorderAcetylcholineNicotinic acetylcholine receptor
The present invention relates to novel geminal substituted quinuclidine amide compounds, and pharmaceutical compositions of the same, that are suitable as agonists or partial agonists of α7-nAChR, and methods of preparing these compounds and compositions, and the use of these compounds and compositions in methods of maintaining, treating and / or improving cognitive function. In particular, methods of administering the compound or composition to a patient in need thereof, for example a patient with a cognitive deficiency and / or a desire to enhance cognitive function, that may derive a benefit therefrom.
Owner:AXOVANT SCI GMBH
Substituted 6-membered aryl or heteroaryl allosteric modulators of nicotinic acetylcholine receptors
The present disclosure relates to compounds of formula (I) that are useful as modulators of α7 nAChR, compositions comprising such compounds, and the use of such compounds for preventing, treating, or ameliorating disease, particularly disorders of the central nervous system such as cognitive impairments in Alzheimer's disease, Parkinson's disease, and schizophrenia, as well as for L-DOPA induced-dyskinesia and inflammation.
Owner:MERCK SHARP & DOHME LLC
Substituted bicyclic heteroaryl allosteric modulators of nicotinic acetylcholine receptors
Owner:MERCK SHARP & DOHME LLC
3-quinuclidinyl amino-substituted biaryl derivatives
InactiveUS20070275975A1Improve cognitive functionLimited abilityBiocideNervous disorderStereochemistryΑ7 nachr
Compounds of formula (I) wherein A is N or N+—O−; n is 0, 1, or 2; Y is O, S, —NH—, and —N-alkyl-; Ar1 is both 6-membered aromatic rings; Ar2 is 5- or 6-membered aromatic rings with a —NR8R9 group, as defined herein. The compounds are useful in treating conditions or disorders prevented by or ameliorated by α7 nAChR ligands. Also disclosed are pharmaceutical compositions having compounds of formula (I) and methods for using such compounds and compositions.
Owner:JI JIANGUO +2
Cyclic compounds as receptor modulating therapeutics and methods and uses thereof
The present invention relates generally to chemical compounds of formula (I) and methods for their use and preparation. In particular, the invention relates to chemical compounds which are useful in relation to the treatment of diseases, disorders or conditions which would benefit from the modulation of the alpha 7 nicotinic acetylcholine receptor (α7 nAChR), such as anxiety, depression and stress related disorders.
Owner:BONOMICS LTD
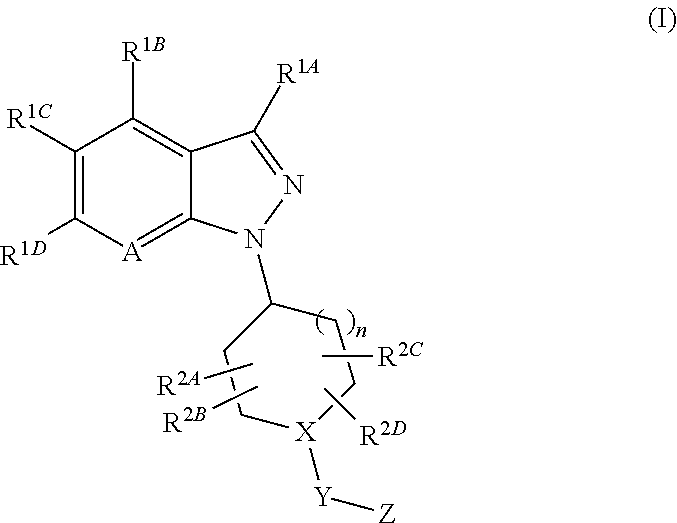
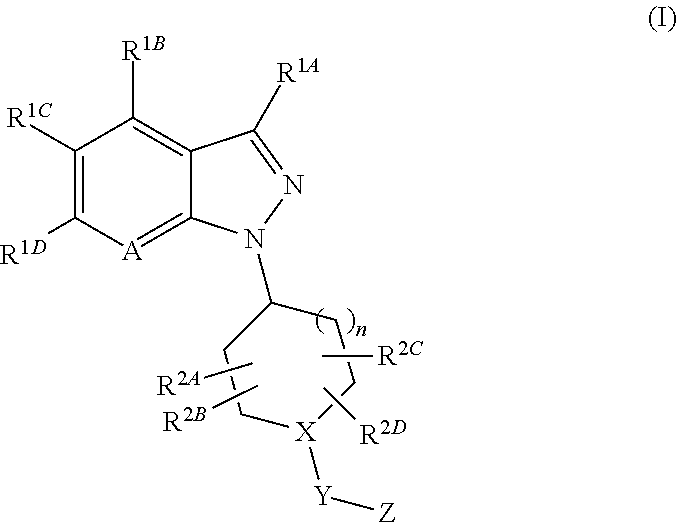
1-substituted indazole derivative
A medicament for treating diseases associated with cholinergic properties in the central nervous system (CNS) and / or peripheral nervous system (PNS), diseases associated with smooth muscle contraction, endocrine disorders, neurodegenerative disorders and the like, which comprises a compound of Formula (I):wherein A is CR1E or a nitrogen atom, X—Y—Z is N—CO—NR3AR3B and the like, R1A to R1E are each independently a hydrogen atom and the like, R2A to R2D are each independently a hydrogen atom and the like, R3A and R3B are each independently an optionally-substituted C3-10 cycloalkyl and the like, and n is 1 or 2or a pharmaceutically acceptable salt thereof, which exhibits potent modulatory-effects on the activity of α7 nicotinic acetylcholine receptor (α7 nAChR).
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Geminal substituted aminobenzisoxazole compounds as agonists of α7-nicotinic acetylcholine receptors
InactiveUS10428062B2Positive effect on clinical functionMinimizing progressionNervous disorderOrganic chemistryMedicineNicotinic Acetylcholine Receptor Agonist
Owner:AXOVANT SCI GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
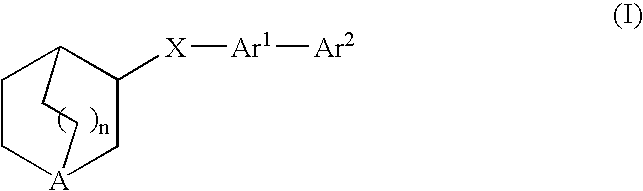
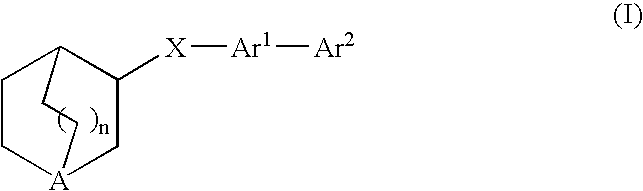

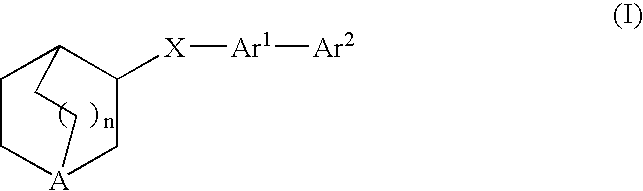
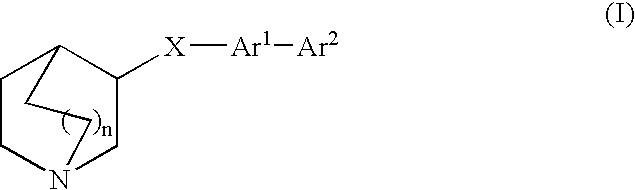
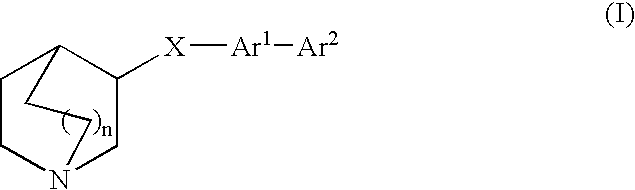
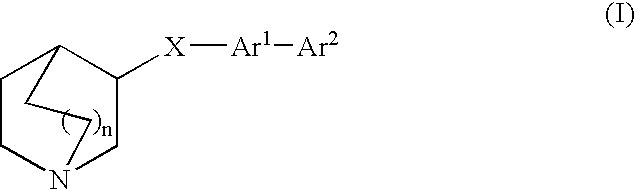
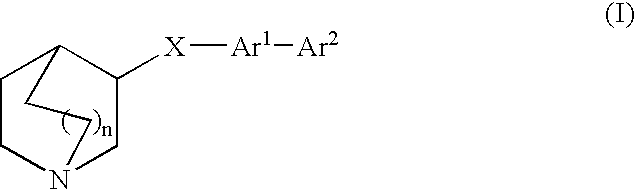
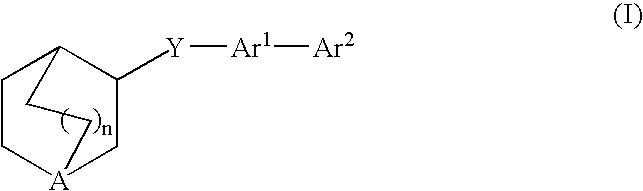
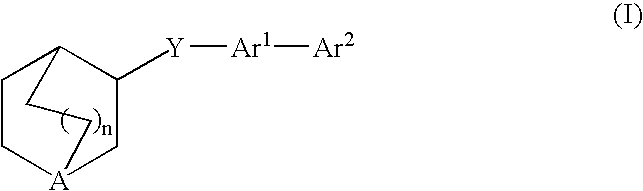
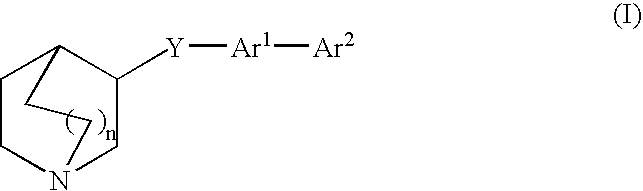
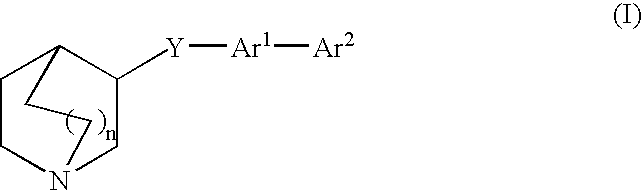





![Crystalline fumarate salts of 1-azabicyclo[2.2.2]oct substituted furo[2,3-c]pyridinyl carboxamide and compositions and preparations thereof Crystalline fumarate salts of 1-azabicyclo[2.2.2]oct substituted furo[2,3-c]pyridinyl carboxamide and compositions and preparations thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0a4e3aee-c307-449b-bdba-31835a84289c/US20050165047A1-20050728-C00001.png)
![Crystalline fumarate salts of 1-azabicyclo[2.2.2]oct substituted furo[2,3-c]pyridinyl carboxamide and compositions and preparations thereof Crystalline fumarate salts of 1-azabicyclo[2.2.2]oct substituted furo[2,3-c]pyridinyl carboxamide and compositions and preparations thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0a4e3aee-c307-449b-bdba-31835a84289c/US20050165047A1-20050728-C00002.png)
![Crystalline fumarate salts of 1-azabicyclo[2.2.2]oct substituted furo[2,3-c]pyridinyl carboxamide and compositions and preparations thereof Crystalline fumarate salts of 1-azabicyclo[2.2.2]oct substituted furo[2,3-c]pyridinyl carboxamide and compositions and preparations thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0a4e3aee-c307-449b-bdba-31835a84289c/US20050165047A1-20050728-C00003.png)