Method and apparatus for manufacturing, filling and packaging medical devices and medical containers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
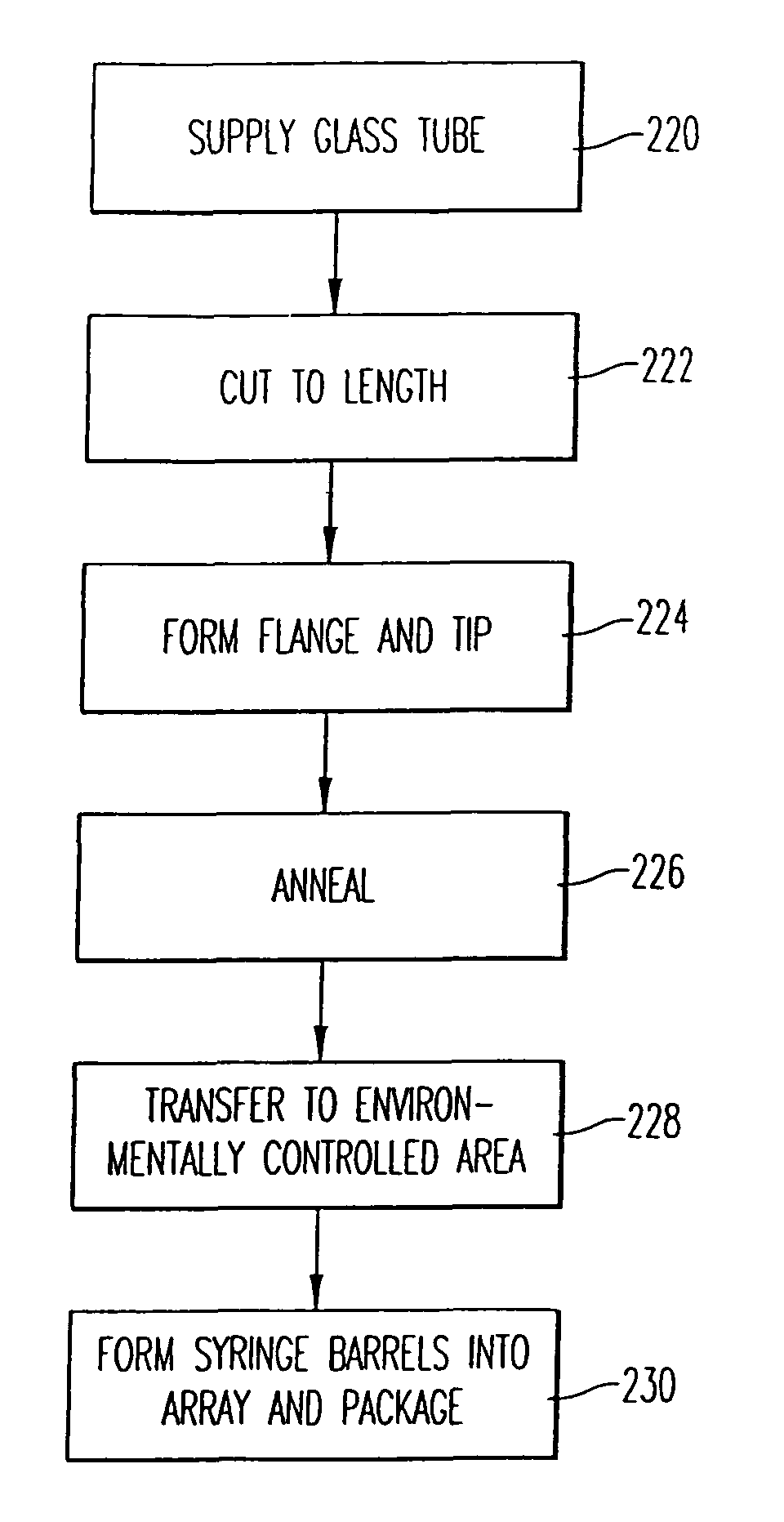
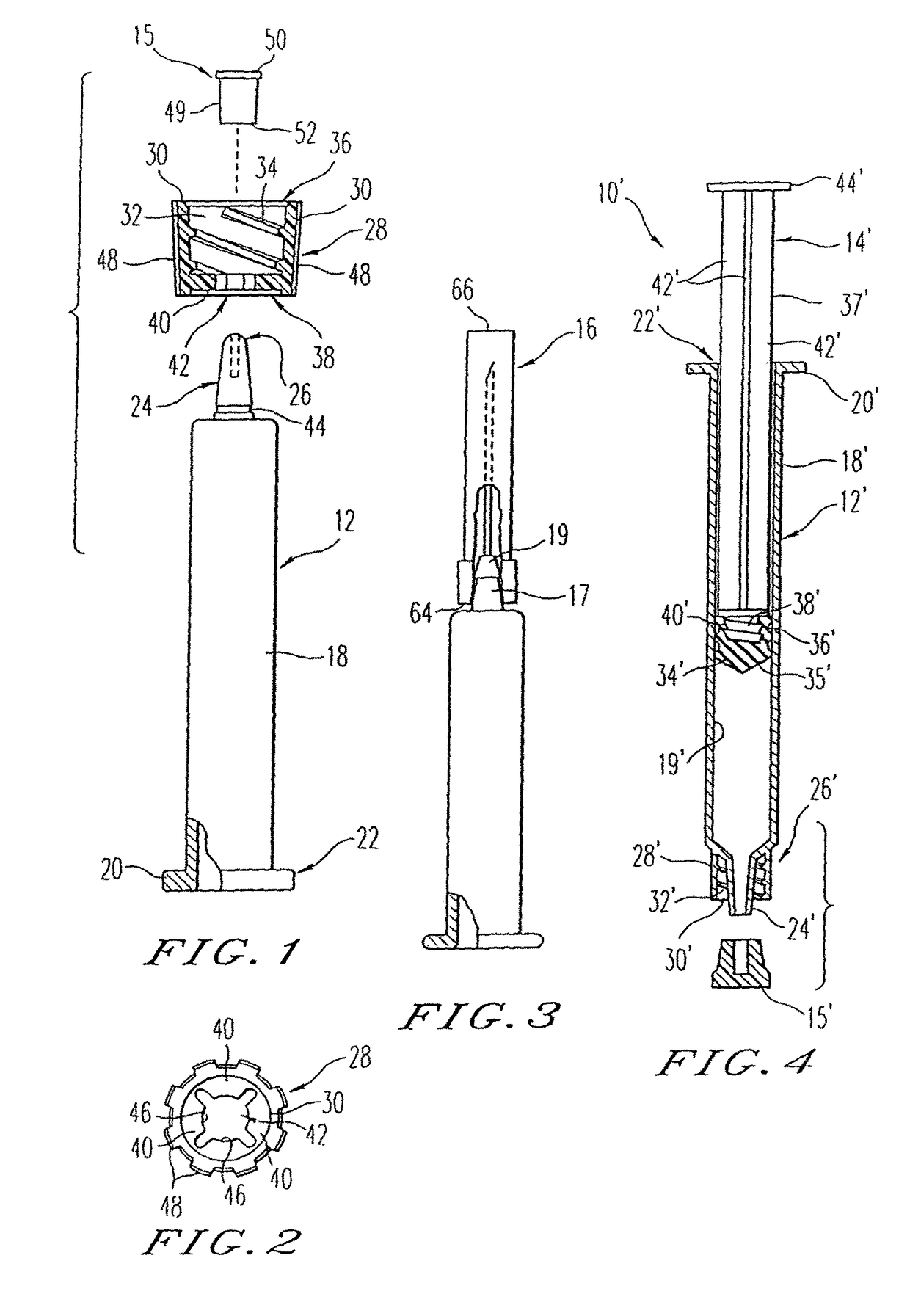
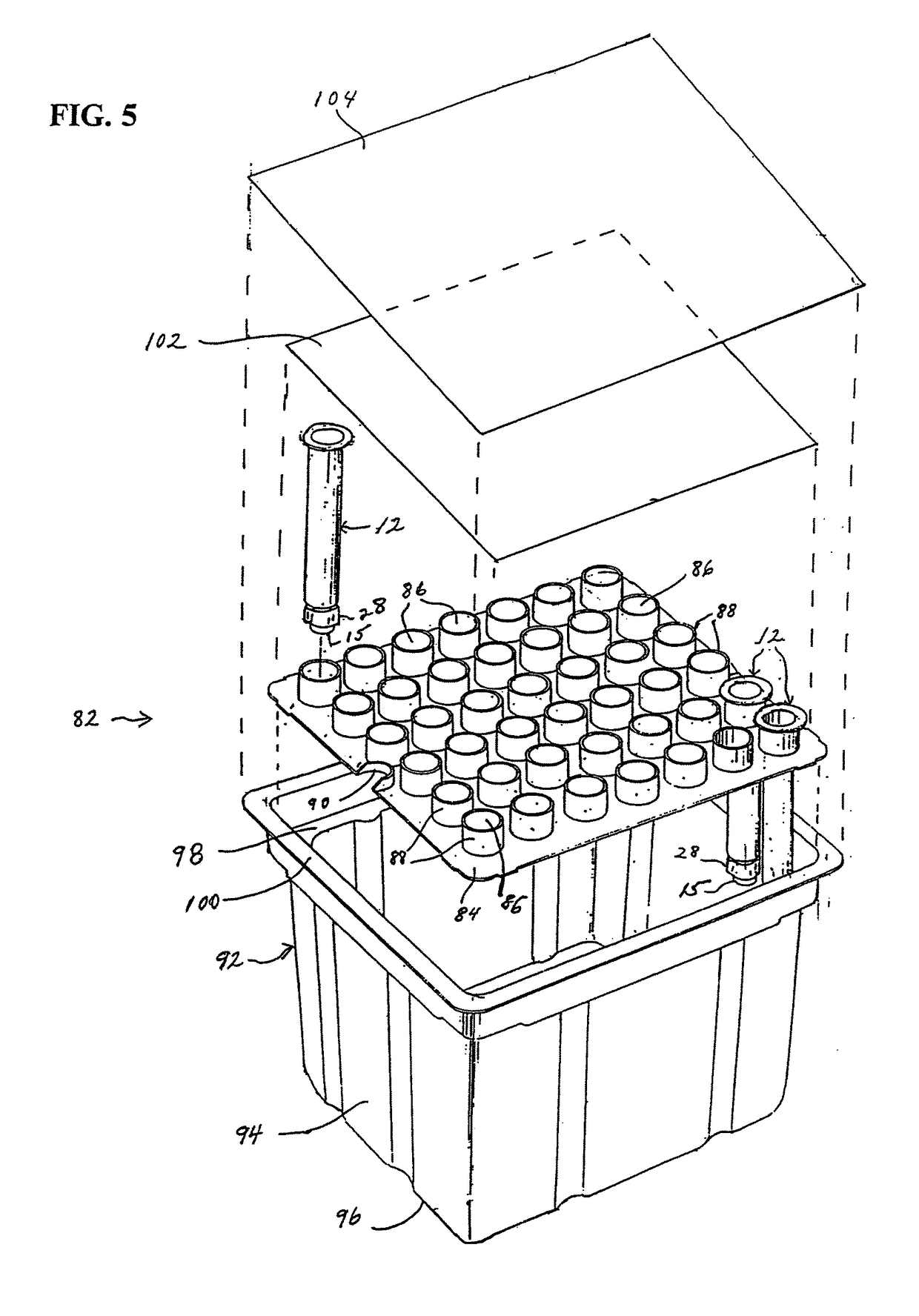
[0034]The present invention is directed to a method and apparatus for manufacturing and thereafter assembling and packaging medical containers, drug delivery devices and drug containers, such as vials, syringe barrels and prefilled syringes, in a clean, environmentally controlled area. As used herein, medical containers for containing and / or dispensing substances include vials and injection devices such as syringes. In addition, as used herein, a substance includes, for example, water, saline solutions, flush solutions and contrasting agents, pharmaceutical agents and vaccines in either a dry state or liquid state. The medical containers can be syringe barrels formed from a base material such as glass or plastic. The syringe barrels are used to assemble a syringe 10 as shown in FIG. 1. Although embodiments of the invention are disclosed as a hypodermic syringe assembly, it is within the purview of the present invention to include various other drug containers, such as plastic or gla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com