Particles incorporating surfactants for pulmonary drug delivery
a technology of surfactants and particle containing substances, which is applied in the direction of peptide/protein ingredients, spray delivery, prosthesis, etc., can solve the problems of poor control, local toxic effects, phagocytosis of lung macrophages, etc., and achieves the effects of avoiding phagocytosis, effective carriers, and improving aerosolization properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Aerodynamically Light Poly[(p-carboxyphenoxy)-hexane anhydride] ("PCPH") Particles
Aerodynamically light poly[(p-carboxyphenoxy)-hexane anhydride] ("PCPH") particles were synthesized as follows. 100 mg PCPH (MW.about.25,000) was dissolved in 3.0 mL methylene chloride. To this clear solution was added 5.0 mL 1% w / v aqueous polyvinyl alcohol (PVA, MW .about.25,000, 88 mole % hydrolyzed) saturated with methylene chloride, and the mixture was vortexed (Vortex Genie 2, Fisher Scientific) at maximum speed for one minute. The resulting milky-white emulsion was poured into a beaker containing 95 mL 1% PVA and homogenized (Silverson Homogenizers) at 6000 RPM for one minute using a 0.75 inch tip. After homogenization, the mixture was stirred with a magnetic stirring bar and the methylene chloride quickly extracted from the polymer particles by adding 2 mL isopropyl alcohol. The mixture was continued to stir for 35 minutes to allow complete hardening of the microparticles. The hard...
example 2
Synthesis of Spray-Dried Particles
Aerodynamically Light Particles Containing Polymer and Drug Soluble in Common Solvent
Aerodynamically light 50:50 PLGA particles were prepared by spray drying with testosterone encapsulated within the particles according to the following procedures. 2.0 g poly (D,L-lactic-co-glycolic acid) with a molar ratio of 50:50 (PLGA 50:50, Resomer RG503, B.I. Chemicals, Montvale, N.J.) and 0.50 g testosterone (Sigma Chemical Co., St. Louis, Mo.) are completely dissolved in 100 mL dichloromethane at room temperature. The mixture is subsequently spray-dried through a 0.5 mm nozzle at a flow rate of 5 mL / min using a Buchi laboratory spray-drier (model 190, Buchi, Germany). The flow rate of compressed air is 700 nl. The inlet temperature is set to 30.degree. C. and the outlet temperature to 25.degree. C. The aspirator is set to achieve a vacuum of -20 to -25 bar. The yield is 51% and the mean particle size is approximately 5 .mu.m. Larger particle size can be achi...
example 3
Fabrication of PLGA microspheres by a Double Emulsion Process Which Encapsulate a Model High-Molecular-Weight Drug, FITC-Dextran.
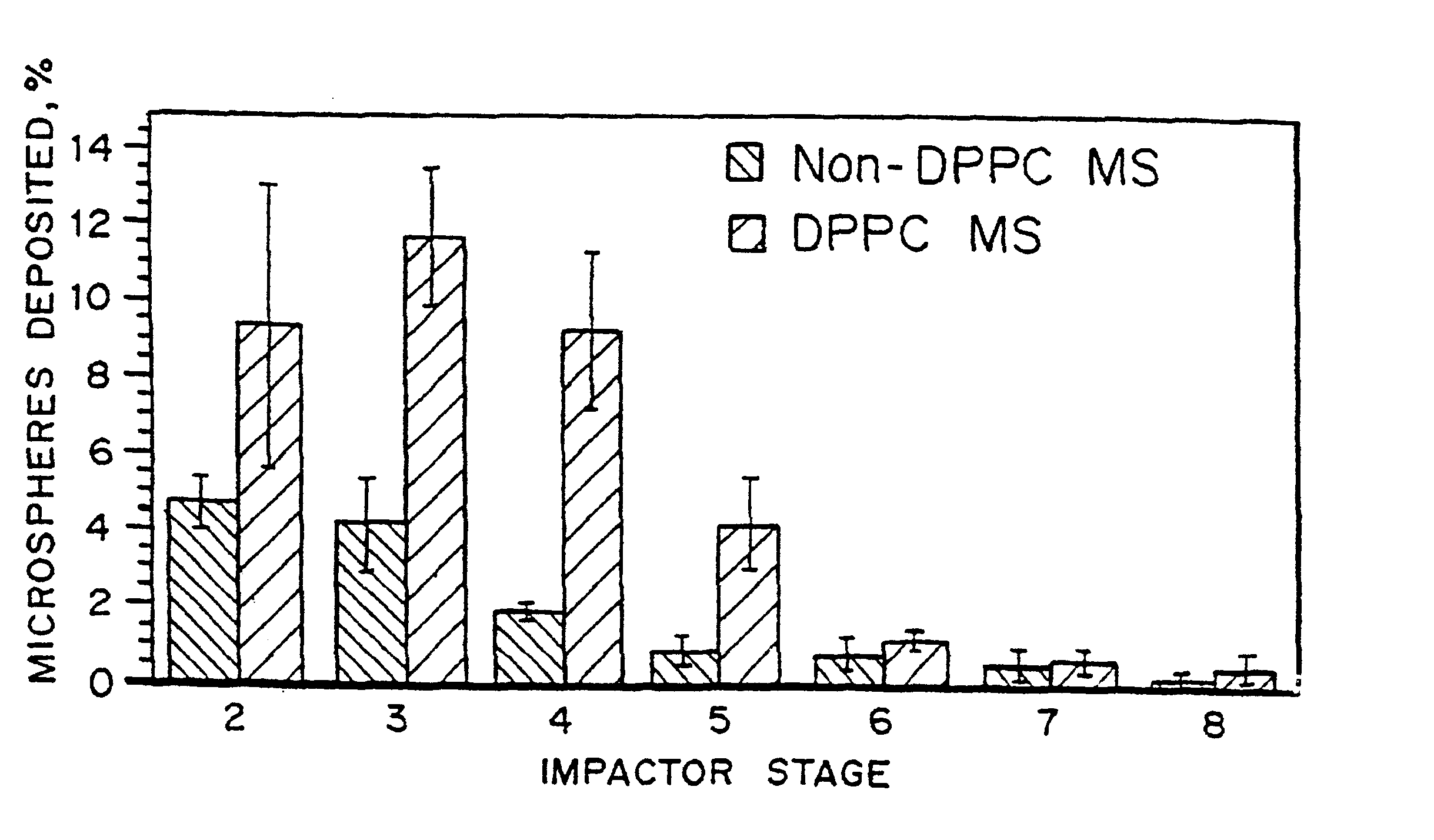
Scanning electron microscopy "SEM" photographs showing surface morphology of microspheres (MS) made by the double emulsion process with and without the lung surfactant, DPPC were obtained. By SEM, the microspheres made with and without DPPC by the double emulsion process had very similar surface characteristics and size distribution, as confirmed by size distribution measurements, shown below in Table 1.
The efficient entrapment of DPPC within microspheres (83% of theoretical .+-.11% standard deviation, n=6) was confirmed by dissolving an aliquot of MS in chloroform and detecting the DPPC concentration in solution by the Stewart Assay, as shown in Table 1. Particles made by double emulsion with DPPC are easily resuspended in aqueous solution after lyophilization and are lump-free when dry as determined by light microscopy. Particles made by the double emuls...
PUM
| Property | Measurement | Unit |
|---|---|---|
| aerodynamic diameter | aaaaa | aaaaa |
| aerodynamic diameter | aaaaa | aaaaa |
| aerodynamic diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com