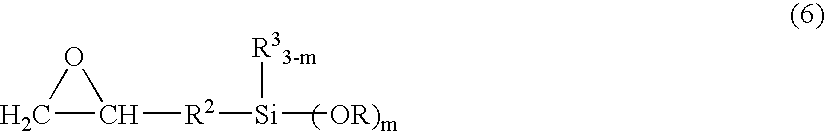Curing resin, method for producing same and curing resin composition
a technology of curing resin and curing mixture, which is applied in the direction of organic non-macromolecular adhesives, cellulose adhesives, adhesive types, etc., can solve the problems of insufficient blending ratio of components of various adhesives and sealants, difficulty in achieving the effect of steric hindrance, and insufficient blending ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 6
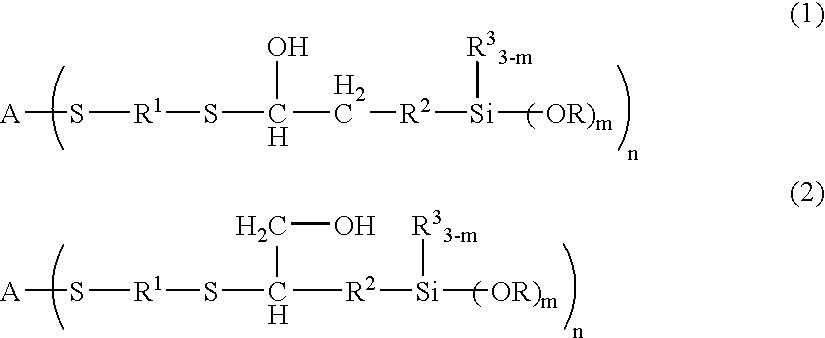
[0199]100 g of 1,2-ethanedithiol, 248 g of TSL8350 (trade name, manufactured by GE Toshiba Silicones, γ-glycidoxypropyltrimethoxysilane) and 1.0 g of triethylamine were placed in a reaction container, and the mixture was allowed to react under nitrogen atmosphere at 50° C. for 7 days, and unreacted 1,2-ethanedithiol was removed at 100° C. under reduced pressure, to give a synthetic product B-3. Then, 600 g of polyallylether having allyl groups at both terminals (number-average molecular weight of 8,000) and 45 g of the synthetic product B-3 were placed in a reaction container and heated to 90° C. Then, a mixture solution of 2 g of AIBN and 10 g of toluene was added dropwise thereto over a period of 2 hours. The mixture was further allowed to react at the same temperature for 1 hour, to give a curable resin.
example 1
Preparative Example 1
(Preparation of Amino Group-Containing hydrolysable alkoxysilane by Michael Addition Reaction)
[0205]100.1 g of ethyl acrylate and 163.3 g of KBM902 (trade name, manufactured by Shin-Etsu Chemical, γ-aminopropylmethyldimethoxysilane) were placed in a reaction container, and the mixture was allowed to react while stirred under nitrogen atmosphere at 23° C. for 7 days, to give a reaction product (1-A). Similarly, 200.2 g of ethyl acrylate and 222.4 g of KBM603 were placed in a reaction container, and the mixture was allowed to react while stirred under nitrogen atmosphere at 23° C. for 7 days, to give a reaction product (1-B).
example 2
Preparative Example 2
(Preparation of Amino Group-Containing Compound (e) by Michael Addition Reaction)
[0206]100.1 g of ethyl acrylate and 57.1 g of allylamine were placed in a reaction container, and the mixture was allowed to react while stirred under nitrogen atmosphere at 50° C. for 7 days, to give a reaction product 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com