DHEA composition and method
a technology of composition and method, applied in the field of dhea, can solve problems such as variability in bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of DHEA and Polymorphs I and II
[0112]Synthesis of DHEA. DHEA was prepared from DHEA acetate (obtained from Diosynth, Chicago, Ill. or Berlichem, Montville, N.J.) by saponification using potassium carbonate in methanol. The product was dissolved in 6 parts methanol at reflux, and charcoal was added and removed by filtration. The methanol was evaporated until a volume of 3 parts remained, and the solution was cooled to 15° C., maintained at this temperature for 1 hour, and filtered. The wet product was refluxed with 8.5 parts of water to remove the methanol, filtered, and dried under vacuum at 90° C. The loss on drying specification for the final product was ≦0.5%, and the specification for residual methanol was ≦0.01%.
[0113]Preparation of Form I. Thirty grams of DHEA, as prepared above, were placed in a 500 mL flask under a nitrogen atmosphere. Anhydrous 2-propanol (isopropanol) was added until all the DHEA dissolved. The resulting solution was stirred under a nitrogen fl...
example 2
Pharmacokinetics of Orally Administered DHEA Formulations: Single Dose Study
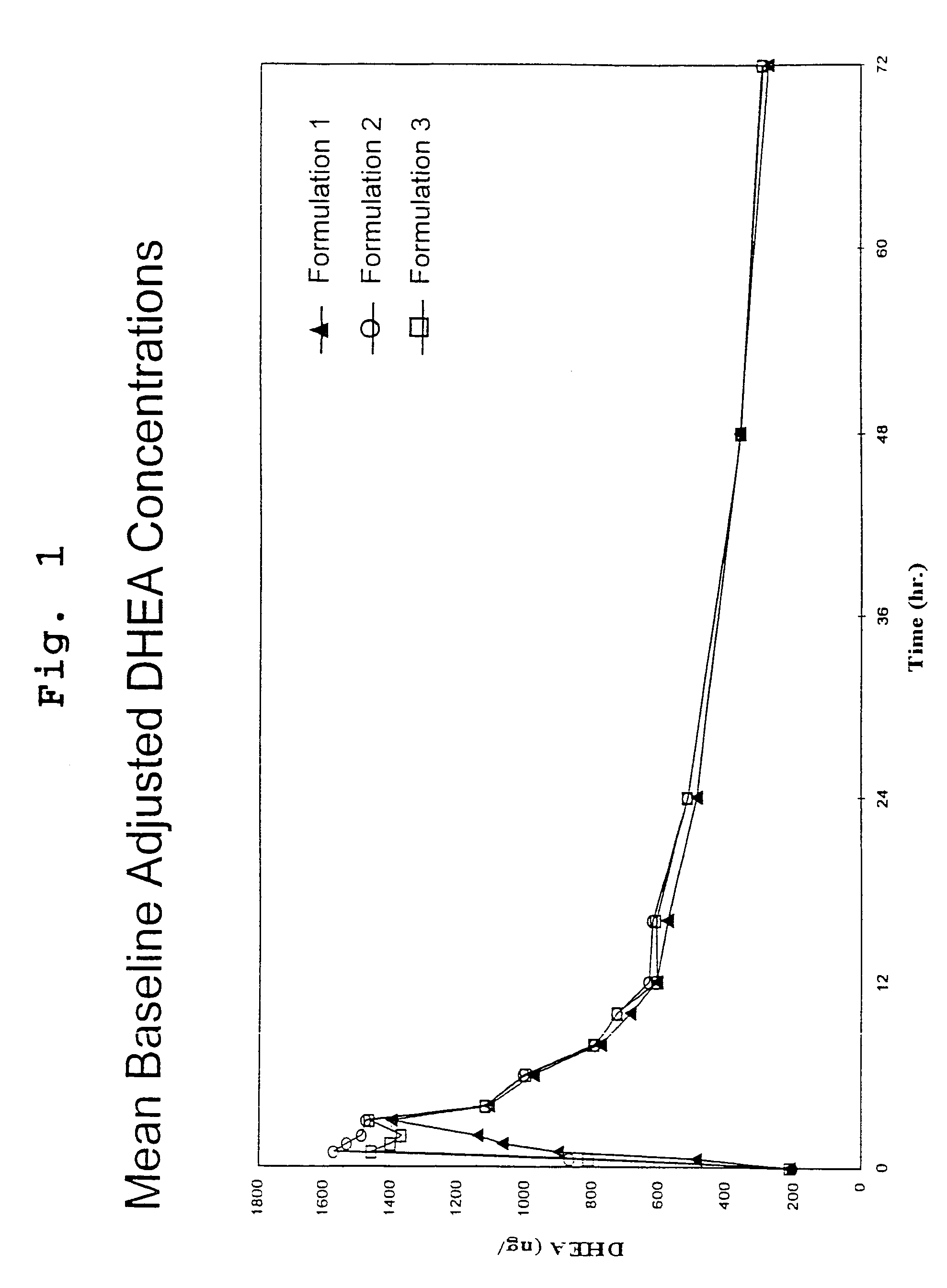
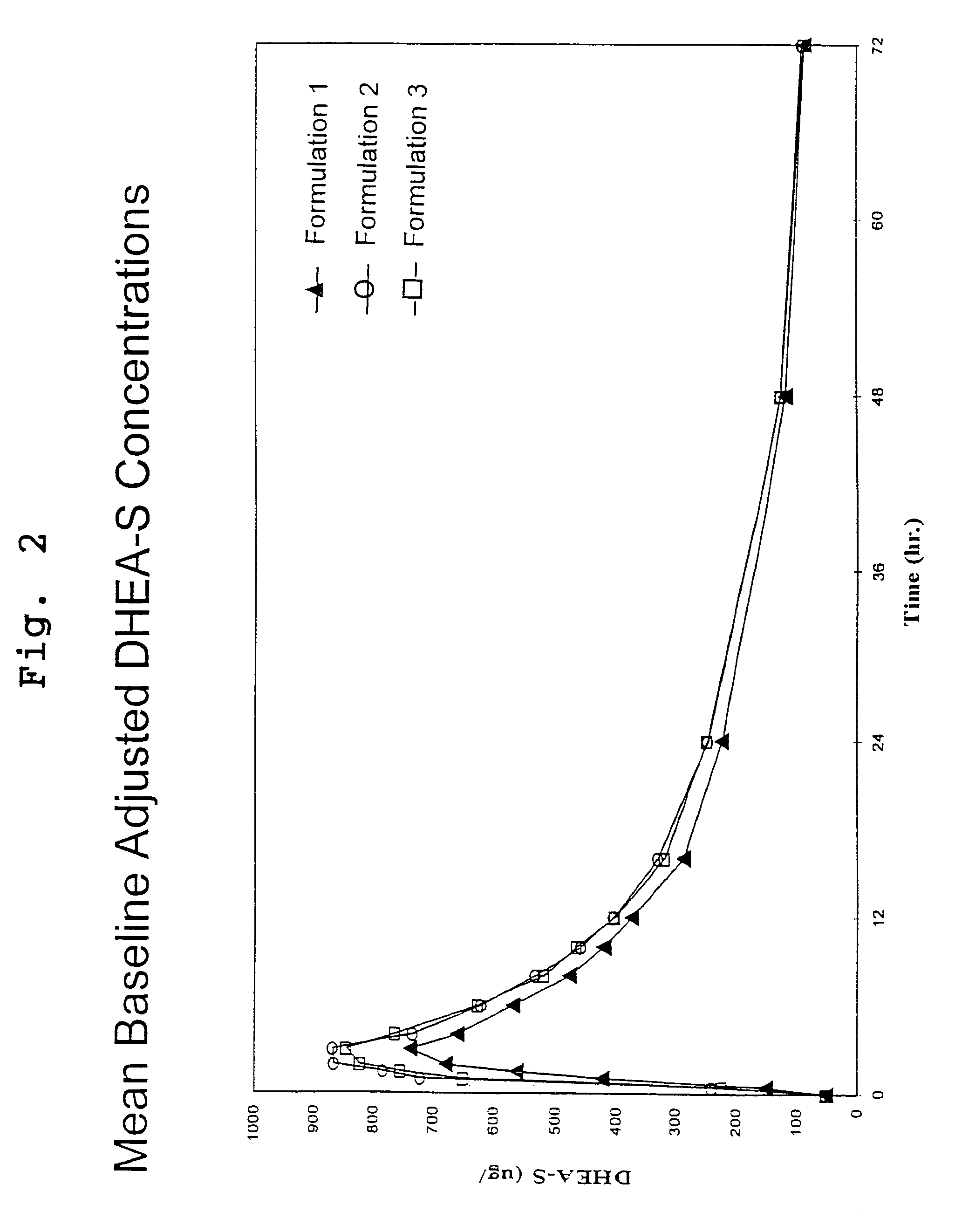
[0115]This study was an open-label, randomized, three period crossover pharmacokinetic study in 34 healthy postmenopausal women. Subjects were contacted the evening prior to each dosing visit and reminded to begin an overnight fast, with nothing to eat or drink (no water permitted) for 10 hours prior to dosing. Serum samples for measurement of DHEA and DHEA-S were drawn thirty minutes prior to dosing and at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 24, 48 and 72 hours after the subjects had received a 200 mg oral dose of DHEA (4 capsules of formulation 1 or 2) with 8 oz. of water. Each dosing period was followed by a 7 day washout period before the next administration.
[0116]DHEA levels were determined by radioimmunoassay (RIA) at Endocrine Sciences Inc., after non-polar solvent extraction. Method validation data demonstrated a recovery range of 92–99%, a limit of detection (LOD) of 18.9 ng / dL, a limit of qu...
example 3
Pharmacokinetics of Orally Administered DHEA Formulations: Multiple Dose Study
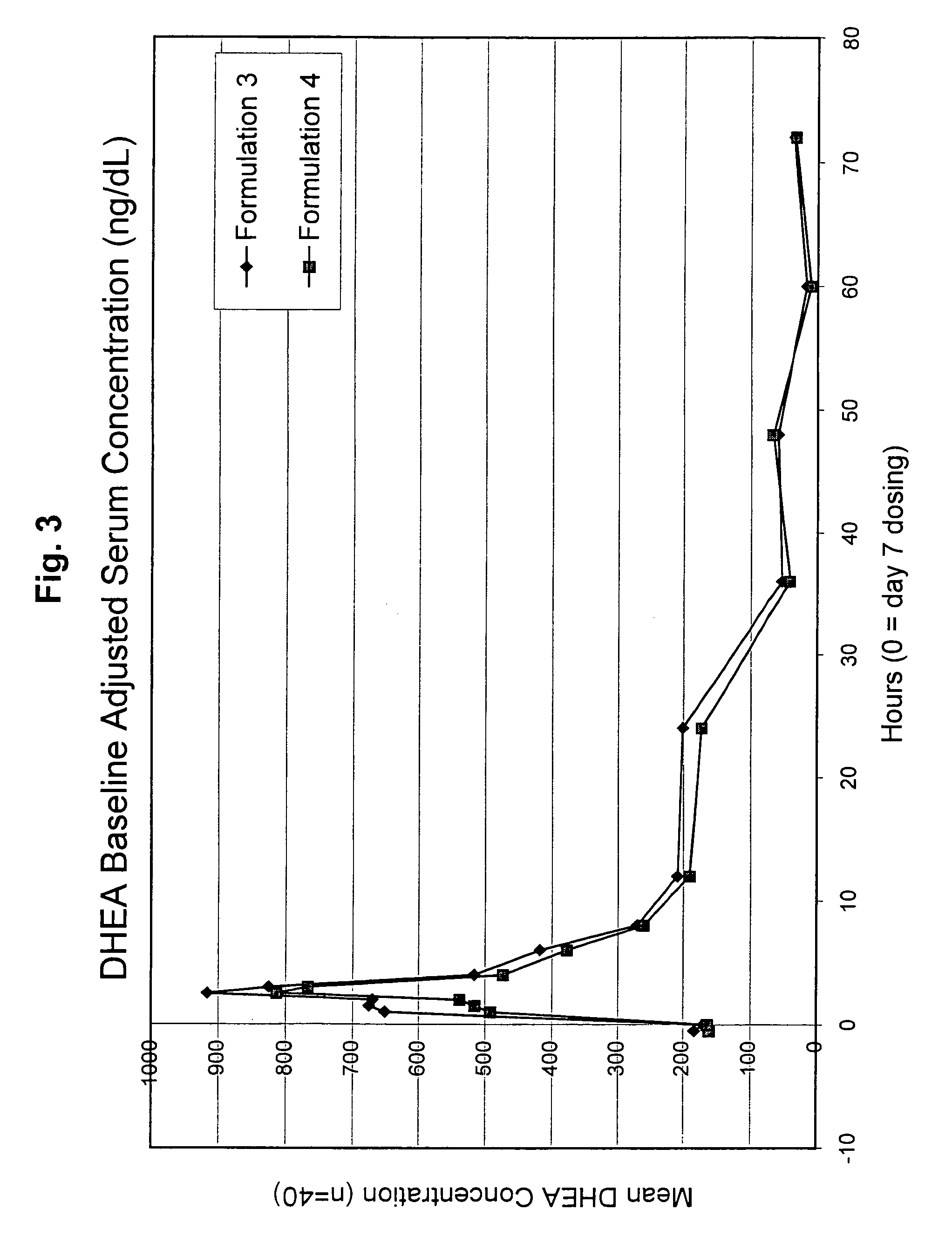
[0122]This study was an open-label, randomized, steady-state, two-treatment cross-over study of DHEA pharmacokinetics / pharmacodynamics in healthy postmenopausal females. DHEA was administered on each of 7 days of two study periods, with an intervening 7-day washout period (Days 1 to 7 and Days 15 to 21). Subjects received a single 200 mg oral dose (four 50 mg capsules) of DHEA at the same time each morning for the 7 days of each study period. Subjects were instructed to fast for 10 hours prior to each DHEA dose. Each capsule contained 50 mg of DHEA and pharmaceutical excipients (169 mg (Formulation 3) or 152 mg (Formulation 4) lactose, 108 mg corn starch, and 3 mg magnesium stearate) to result in a total capsule fill weight of 330 and 313 mg for formulations 3 and 4, respectively. Subjects were randomized to receive one of the two formulations during each study period. The DHEA polymorph composition of for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com