Solutions for oral dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
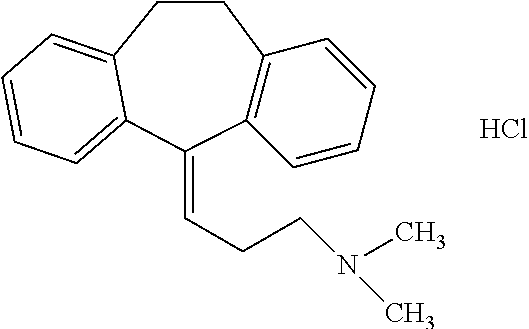
Oral Pharmaceutical Composition Comprising Amitriptyline Hydrochloride
[0074]
Role ofQuantitySr. No.Ingredientsingredients% w / v(mg / mL)1AmitriptylineActive440Hydrochloride(equivalent toamitriptyline)2GlycerolStabilizer / solubilizer383803SucraloseSweetener0.554Suitable FlavorFlavor0.020.25Polysorbate 80Surfactant0.116Purified WaterVehicleq.s to 100 mlQ.S. 1 mL
[0075]Preparation of the formulation above was as follows:[0076]a. Add sufficient quantity of purified water in vessel[0077]b. Add glycerin and mix until homogenously dispersed[0078]c. Add polysorbate 80 and mix until dispersed[0079]d. Add amitriptyline and mix until dissolved[0080]e. Add sucralose and flavor one by one and mix until dissolved[0081]f. Volume make up until it reaches batch size, filter and fill in to suitable packaging
[0082]Those who are skilled in the art can also understand that some variations in the herein described processes for the preparation of liquid compositions of the present invention can be adopted which...
example 2
Study Results of Amitriptyline Liquid Composition
[0084]The oral liquid pharmaceutical composition prepared according to Example 1 exhibits unexpected stability profile when tested after six months:
TestStorage condition40° C. ± 2° C. / NMT75% RH25° C. ± 2° C. / 60 ± 5% RHparametersTentative specificationInitial3 M6 M3 M6 MDescriptionA clear colorless to paleCompliesCompliesCompliesCompliesCompliesyellow colored solutionwith characteristic odor.pHBetween 3.5 and 5.54.5 4.7 4.0 4.8 4.2 Assay ofBetween 90.0% and99.50% 95.80% 100.90% 102.90% 100.60% Amitriptyline110.0% of labeledamount.RelatedSubstanceImpurity -ANot more than 0.2%NDBQLBQLBQLBQLImpurity -BNot more than 0.2%0.08%0.07%0.08%0.08%0.08%(RRT(RRT(RRT(RRT(RRT0.78) 0.78) 0.78) 0.78) 0.78) SingleNot more than 0.2%NDBQLBQLBQLBQLMaximumUnknownimpurityTotalNot more than 1.0%0.08%0.07%0.08%0.08%0.08%Impurities
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com