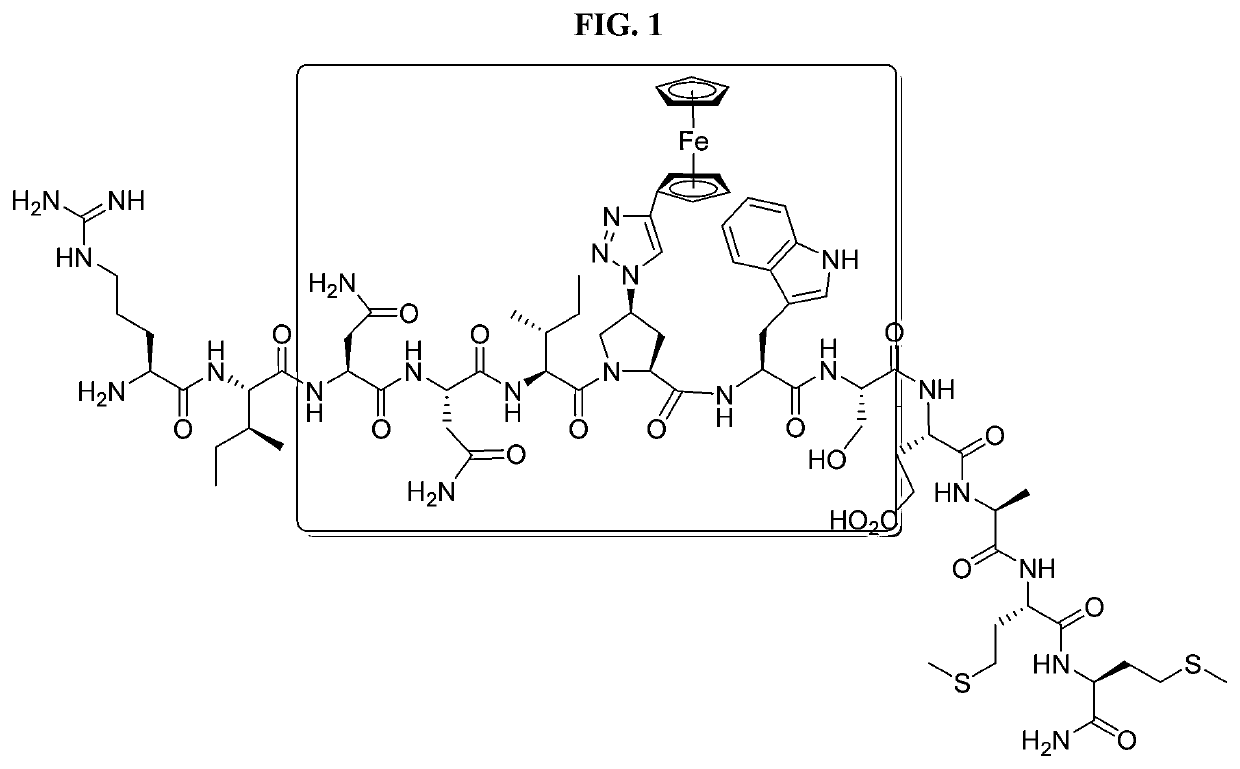
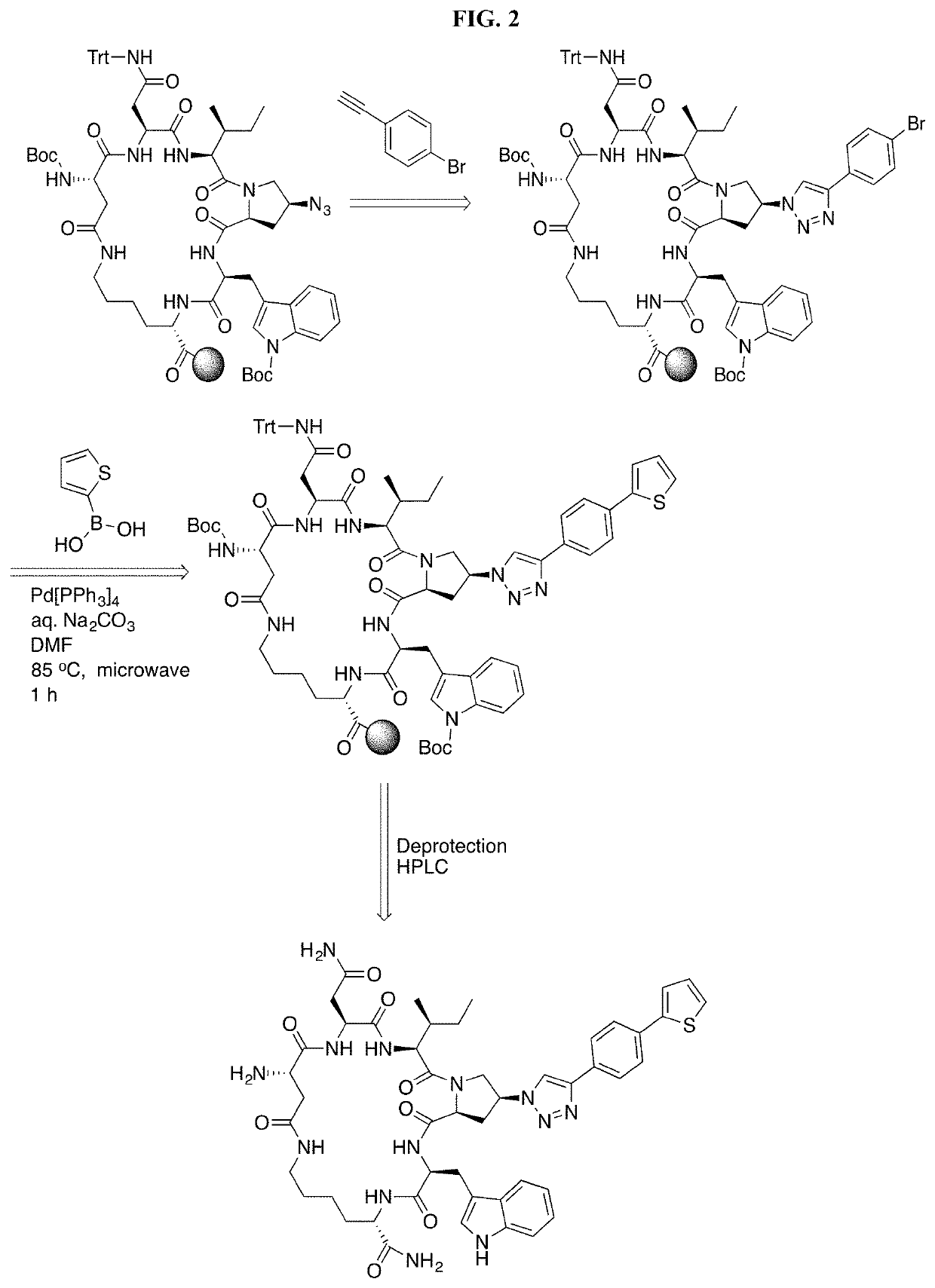
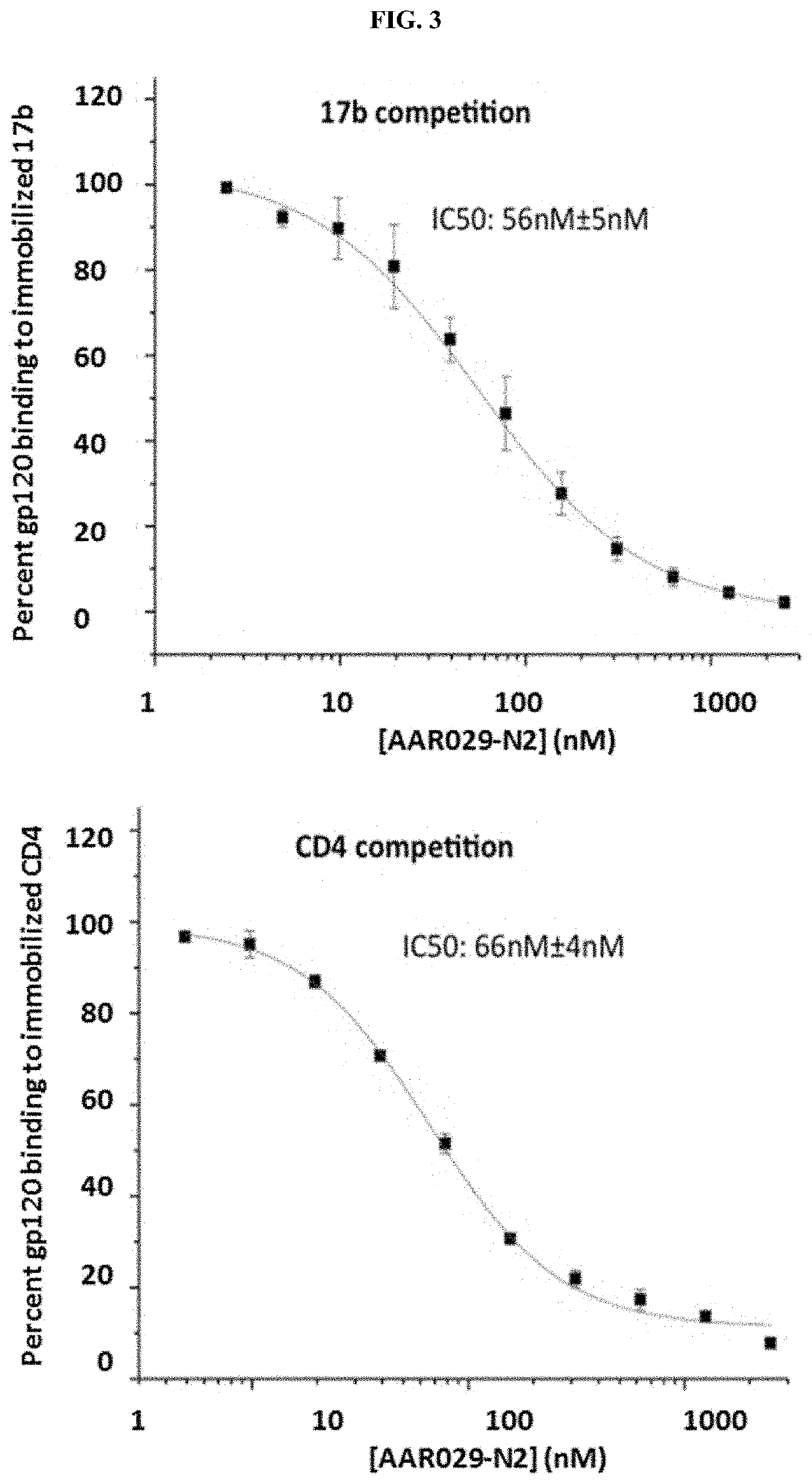
Cyclic Peptide Antiviral Agents and Methods Using Same
a technology of cyclic peptides and antiviral agents, applied in the direction of peptides, peptide/protein ingredients, immunoglobulins, etc., can solve the problems of weak potency and toxicity of existing entry inhibitors, and achieve the effects of reducing the risk of hiv-1 infection, promoting virolysis of a virus, and treating, reducing or preventing hiv-1 infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
ptides
[0181]Cyclic peptides of the invention can be prepared using intramolecular cyclization according to the methods disclosed in International Application Publication No. WO 2016 / 094518, which is incorporated herein by reference in its entirety.
Chemical Synthesis of cPTs (3-38)
[0182]Alkynes a10-a16 were synthesized from the corresponding benzyl bromides a1-a9 following the procedures previously described (Louvel, et al., 2013, J. Med. Chem. 56:9427-40). Alkynes a10-a16 were used in click reactions without purification, because they are volatile and unstable upon storage. Mass validation of all cPTs was performed using Thermo Scientific LTQ XL Ion Trap LC / MS (Table 4).
General Synthesis of Alkynes a9-a16
[0183]To a solution of ethynyltrimethylsilane (40 mmol) in 20 mL THF at 0 ° C., was added i-PrMgCl (2 M solution in THF) dropwise, and the mixture was stirred at 0° C. for 30 min, and allowed to warm up to r.t. for 30 min. CuBr (6 mmol) was added, and the mixture was stirred at r.t....
example 2
us Production, Antiviral Assay and Cytotoxicity Assay
[0185]Pseudoviruses were produced as previously described (Umashankara, et al., 2010, ChemMedChem 5:1871-9). Briefly, HEK 293T cells (3×106) were co-transfected with 4 μg of BaL.01 gp160 plasmid and 8 μg of NL4-3R-E-Luc+ core DNA (obtained from the NIH AIDS Reagent Program), using Polyethyleneimine (PEI) as a transfection vehicle. After 72 hours, the supernatant containing virus was collected and filtered using a 0.45 μm syringe filter (Corning). The supernatant was then loaded on a 10 ml Iodixanol gradient; 6%-20% (Optiprep, Sigma Aldrich) and centrifuged on SW41Ti rotor (Beckman Coulter) at 30,000 RPM for 2 hours at 4° C. Virus samples were pooled from fractions 6 through 9 in 1 ml aliquots and diluted in serum free media, before storage in −80° C. All batches of virus were titrated for infectivity and p24 content immediately after production.
[0186]Pseudoviral infection assays were carried out as previously described (Emileh, et...
example 3
n of HIV-1 gp120 and Soluble CD4 Proteins Reagents
[0189]Escherichia coli strain Stbl2 cells were products of Novagen Inc. (Madison, Wis.). DNA plasmids encoding BaL.01 gp160 and NL4-3R-E-Luc+ were obtained from the NIH AIDS Reagent Program, Division of AIDS, NIAID. pcDNA3.1 vector carrying CD4 was a gift from Navid Madani. 17b IgG was purchased from Strategic Diagnostics Inc. (Newark, Del.). All other reagents used were of the highest analytical grade available.
Expression and Purification of Wild-Type gp120YU-2
[0190]The DNA for gp120YU-2 in pcDNA3.1 vector for transient transfection was purified using a Qiagen MaxiPrep kit (Qiagen) after transforming into Stbl2 competent cells. The purified DNA encoding gp120 YU-2 was transfected into HEK 293F cells according to manufacturer's protocol (Invitrogen). Five days after transfection was initiated, cells were harvested and spun down (3000 RPM), and the supernatant was filtered through 0.2 μm filters. Purification was performed over a 17b ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| MW | aaaaa | aaaaa |
| size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com