Implantable devices for drug delivery with reduced burst release
a technology of implantable devices and drug delivery, which is applied in the direction of drug compositions, peptide/protein ingredients, other domestic articles, etc., can solve the problems of impaired cognitive function, inconvenient or difficult compliance with extended dosing regimens, and many problems, so as to improve the control and tuning of the release rate, the effect of reducing the burst releas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Testing Implants in Canines
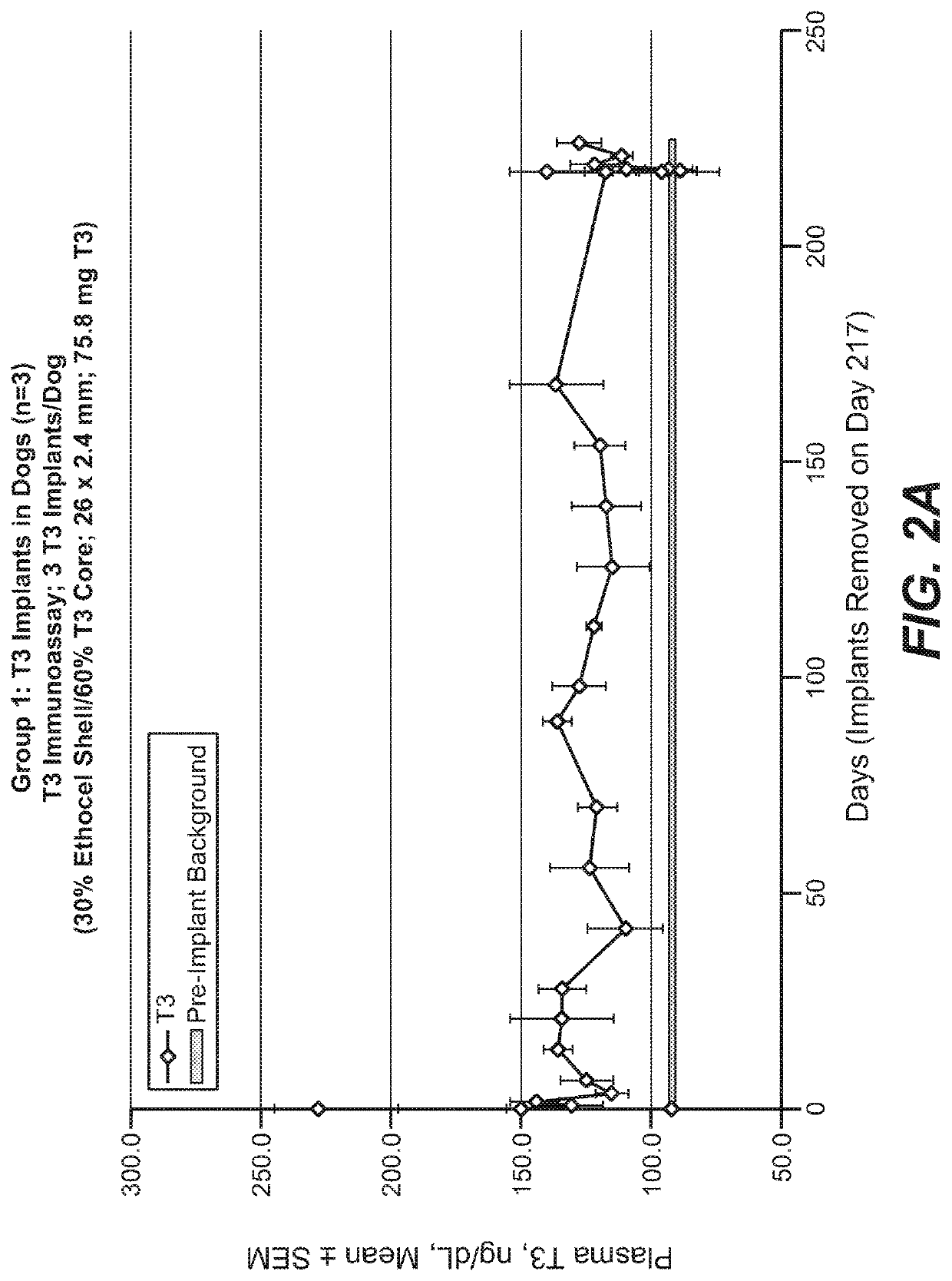
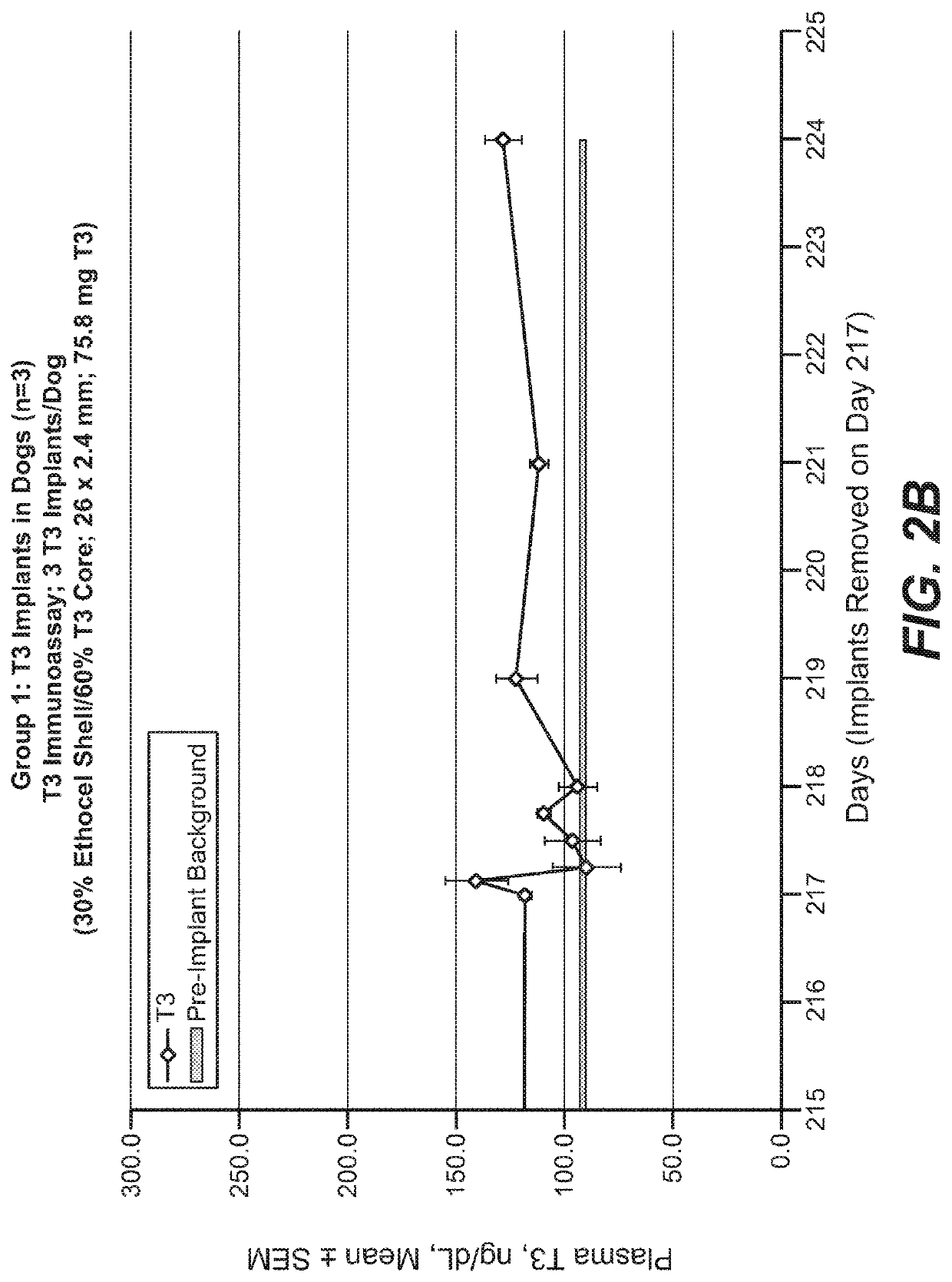
[0259]Implants were prepared by co-extrusion as described above and tested in two groups of dogs, with a third group of dogs serving as control. Each Group 1 dog (n=3) received three implants (30% ethyl cellulose shell / 60% T3 core; 26×2.4 mm; 75.8 mg T3), and were followed for about 8 months, including one week after removal of the implants. Each Group 2 dog (n=3) received three T3 implants (60% ethyl cellulose shell / 60% T3 core) on day 1, three additional T3 implants on day 87, and three additional T3 implants on day 118, for a dose-escalation study. The core of the T3 implants was about 2 mm in diameter, and the shell thickness was about 0.2 mm (twice the shell thickness is added to the core diameter to obtain the 2.4 mm diameter of the T3 implants). Control dogs (n=3) received EVA-based implants containing no thyroid hormones. The first implant was 4 cm in length and 3 mm in diameter; subsequent implants were 6 cm in length and 3 mm in diameter. Implant...
example 2
Testing T3 Implants in Thyroidectomized Rats
[0267]Administration of exogenous T3 results in a decrease in production of endogenous T3 by the thyroid. In order to get around the issue of endogenous T3 confounding the analysis of T3 release from implants, a thyroidectomized rat model with low background T3 was employed.
[0268]Each thyroidectomized rat (n=3) received one T3 implant (60% Ethocel Shell / 60% T3 Core; 40×3 mm) (ETHOCEL is a registered trademark of the Dow Chemical Company, Midland, Mich., United States, for ethyl cellulose polymer). The results are shown in FIG. 7, with comparison to normal rats. T3 release from T3 implants in thyroidectomized rats parallel that seen for these implants in normal rats. The assay upper limits of quantitation were capped at 1200 ng / dL. Implants were washed with ethanol prior to use.
[0269]The data for the normal rats receiving the 60% Ethocel Shell / 60% T3 Core implant is also shown in FIG. 12A and FIG. 12B, in comparison to normal rats receiving...
example 3
Ropinirole Implants in Dogs
[0270]Implants were prepared containing ropinirole. Implants with no shell (“core-only” implant) were prepared with 60% ropinirole in EVA, which were 2.4 mm in diameter and 26 mm in length. Two different sets of implants with shells were prepared. One set of shelled implants was prepared which was 40 mm long and 3 mm in diameter, having the same 2.4-mm-diameter 60% ropinirole in EVA core, and with a 0.3 mm-thick shell having 10% ETHOCEL in an EVA shell (twice the shell thickness is added to the core diameter to obtain the 3 mm diameter of these implants). The second set of shelled implants was similar, but was 60 mm long instead of 40 mm long. The implants were washed with ethanol prior to subdermal implantation. Three groups of male beagle dogs were used, with three dogs in each group. Three dogs received two implants each of the no-shell, core-only implant; three dogs received two implants each of the 40-mm-long implants with shell, and three dogs receiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| wt % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com