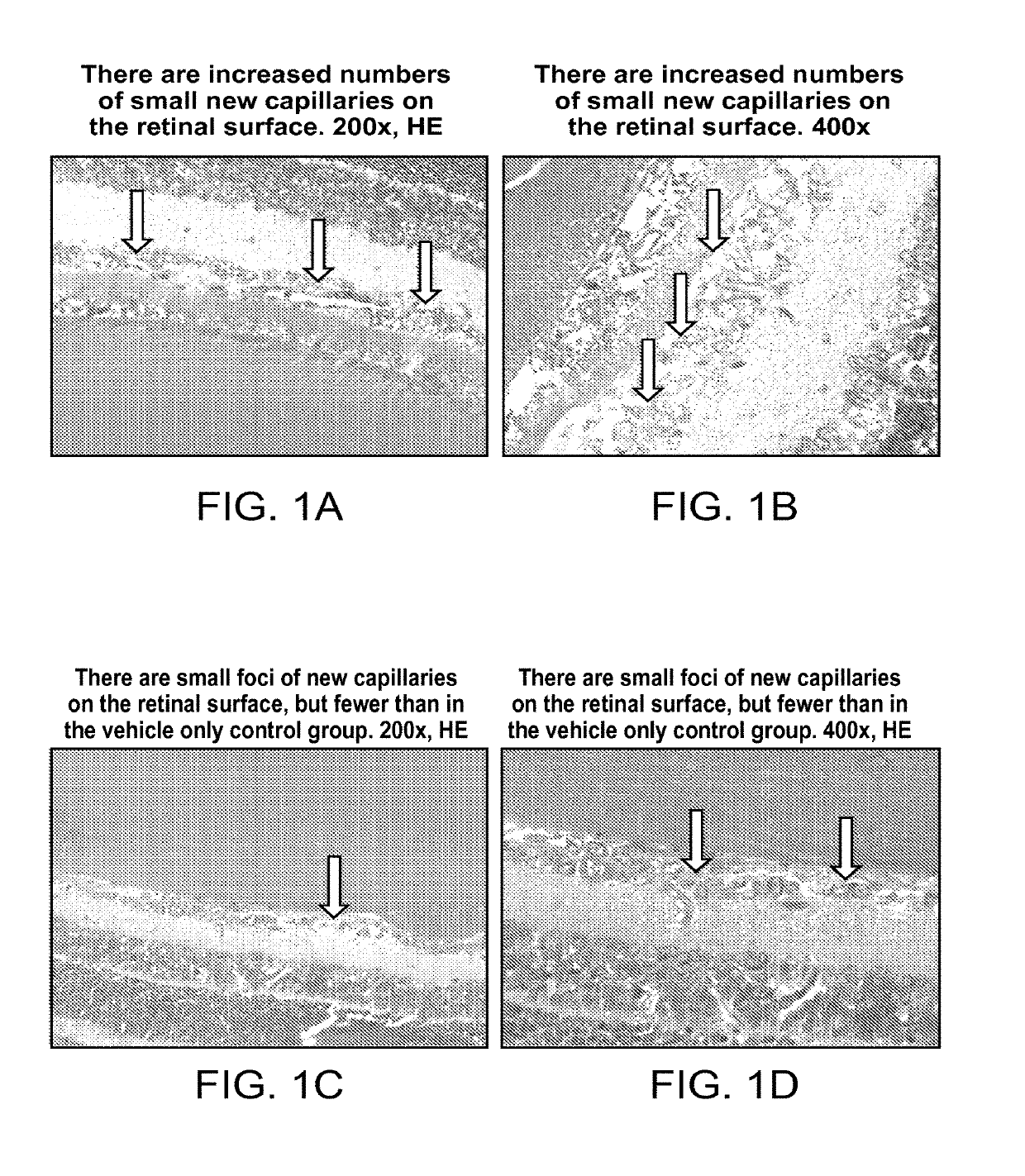
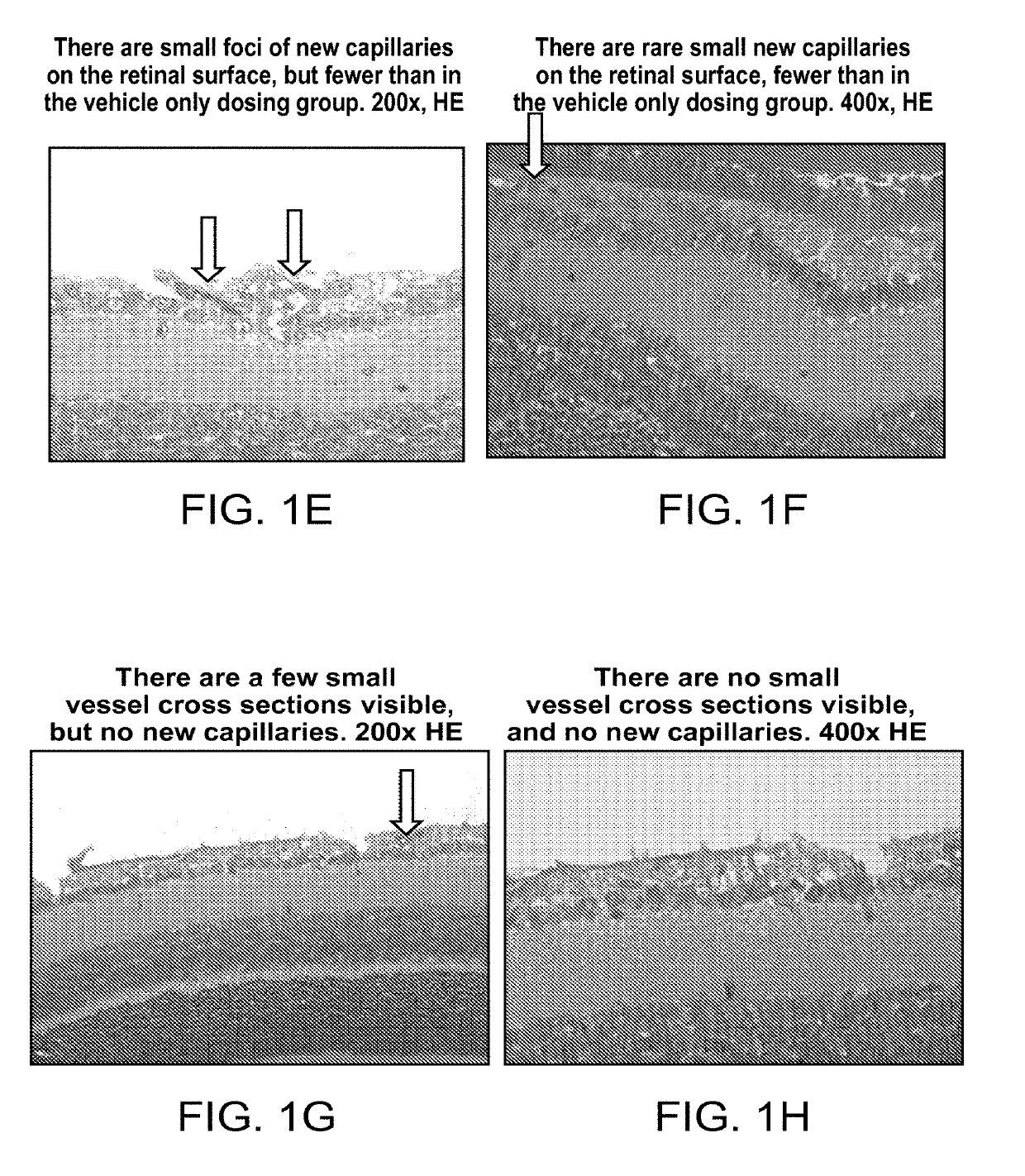
Ophthalmic compositions of rifamycins and uses thereof
a technology of ophthalmic compositions and rifamycin, which is applied in the field of pharmaceutically acceptable compositions, can solve the problems of loss of visual acuity, loss of vision in the center of the visual field (the macula), and difficulty or inability to read or recognize faces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulation of Rifamycin Compounds
[0084]The active ingredient, i.e. rifamipicin, rifabutin, rifapentine, rifalazil or rifaximin is dissolved in saline or water and a surfactant such as polysorbate 80, tween 80 or tween 20 is added and mixed. Further, various additives such as glycerin, xanthan gum, hydroxypropylmethylcellulose (HPMC), cyclodextrin derivatives such as hydroxypropyl-β-cyclodextrin, isotonic agents such as such as sodium chloride, potassium chloride or sodium bisulfate, preservative such as disodium EDTA or methylparaben, anti-oxidant such as ascorbic acid are optionally added and mixed to form a clear solution. The solution is optionally filtered to remove particulate matter and the pH is adjusted by adding an acid such as hydrochloric acid or a base such as sodium chloride to obtain the desired pH.
example 2
[0085]16 different formulations were prepared for topical eye drop application of rifampicin, as disclosed in Tables 1-3. The formulations of Example 1A and Example 6A were used in the following studies.
[0086]The following eye drop formulations were prepared at room temperature (Tables 1 and 2), and Rifampicin was added to the eye drop formulations to the final concentrations listed:
TABLE 1ExampleExampleExampleExampleExample1A2A3A4A5ApH of input buffer (50 mM BoricpH 8.7pH 8.7Acid-Borax)pH of input buffer (100 mMpH 8.3pH 8.3pH 8.3Boric Acid-NaOH)Final concentrations of input 10 mM 10 mM20 mM25 mM25 mMbuffersFinal pH detected after additionpH 7.64pH 7.37pH 8.01pH 8.30of RifampicinNaCl*150 mM150 mM0.9%0.9%0.9%Tween 80* 0.5%0.5%0.5%0.5%0.5%EDTA* 0.1%0.1%0.1%0.1%0.1%Benzalkonium chloride*0.01%0.01% 0.01% 0.01% 0.01% Rifampicin*0.25%0.5%0.5% 1%0.5%*Final concentrations
TABLE 2Exam-Exam-Exam-Exam-Exam-ple 6Aple 7Aple 8Aple 9Aple 10ApH of input bufferpH 8.7pH 8.7pH 8.5pH 8.5pH 8.3(50 mM Bo...
example 3
[0090]Rifampicin was delivered to retina by topical eye drop application. 0.25% rifampicin eye drop formulation was used for the studies. It showed good delivery efficiency, and retina tissue got micro gram concentrations of rifampicin per g of tissue by the eye drop application.
[0091]Four male Sprague-Dawley rats (250-300 g) were used to determine retina exposure levels of rifampicin following applications of eye drops. Two rats received 3 drops of an eye drop formulation (0.25% Rifampicin) which is presented as Example 1A listed in Table 1 in each eye under isoflurane sedation. The remaining two rats received the same applications, but 10 drops, to each eye under isoflurane sedation. A single drop contained 5 micro L of the formulation, and applications of each drop had 30 min interval. After the eye drop application, retinas were extracted under a dissection microscope. In addition, “Non-treatment” negative control was taken, and retina was extracted from two rats without any tre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com