Antibodies targeting bone morphogenetic protein 9 (BMP9) and methods therefor
a technology of bone morphogenetic protein and antibody, applied in the field of antibodies targeting bone morphogenetic protein 9 (bmp9) and methods therefor, can solve the problems of affecting tissue or organ function, so as to reduce the activity of bmp9
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of Recombinant BMP9
[0516]DNA sequence encoding full length hBMP9 protein was cloned an expression vector and confirmed by DNA sequencing. hBMP9 construct was transiently transfected into 293F cell line and the cells were further optimized for hBMP9 protein production. The final production was carried out at 10 L scale and multiple runs. Final harvests were collected when cell viability was >80%. Cell debris in the final harvest were removed by centrifugation and filtration processes. The target hBMP9 protein was purified by using cation exchange chromatography and anion exchange chromatography. Ultrafiltration was used to concentrate hBMP9 protein and to exchange buffer. Quantitation of the protein was determined by Lowry method. Purified hBMP9 protein was analyzed by SDS-PAGE, Western blot and HPLC.
example 2
n of Anti-BMP9 Antibodies by Hybridoma Technology
Mice Immunization and Fusion
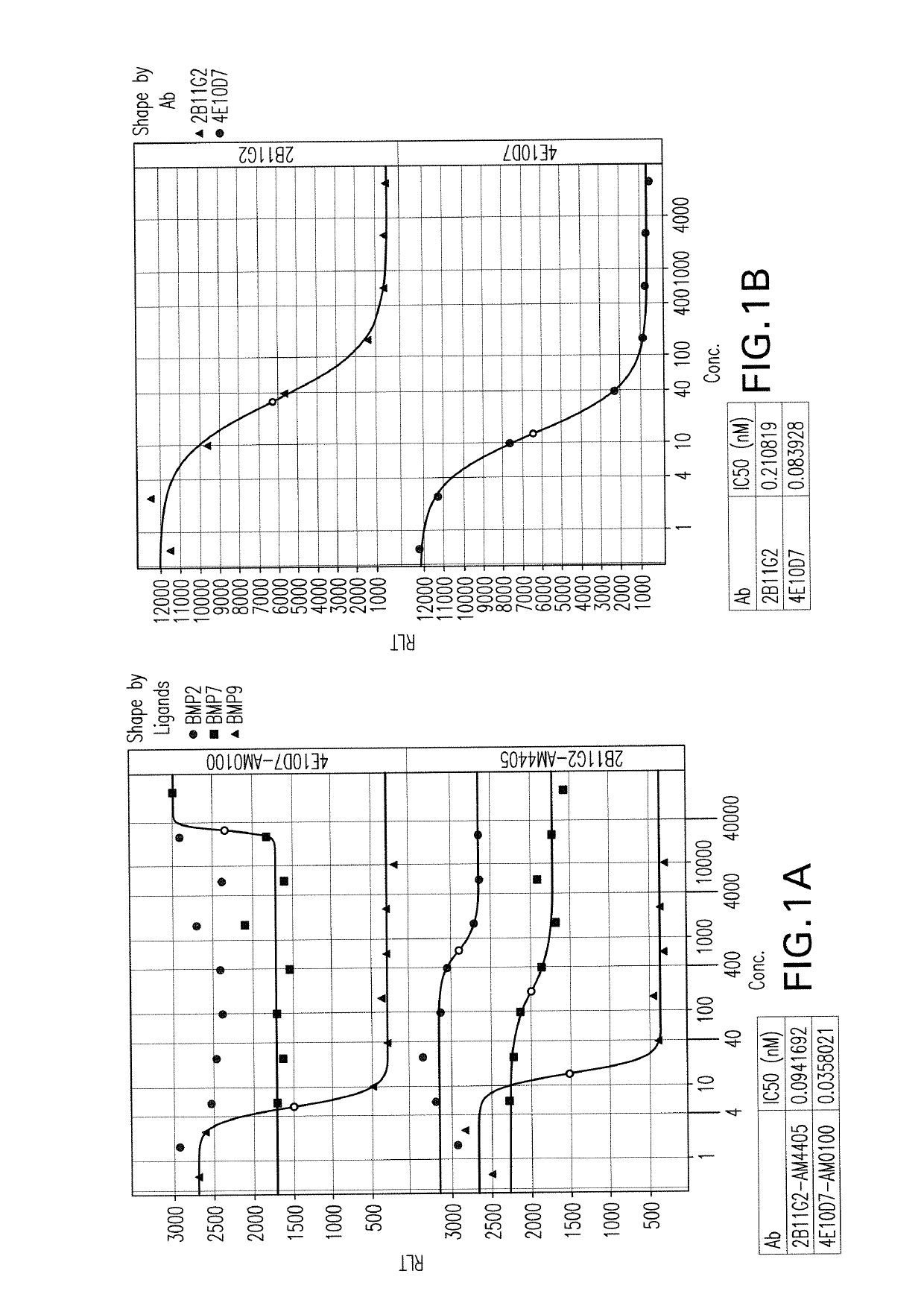
[0517]Ten BALB / c mice were immunized with recombinant protein human BMP9 (huBMP9) by a repetitive procedure involving 4 injections either subcutaneously or interperitoneally of 25-50 ug huBMP9. Spleens of immunized mice were harvested, and isolated splenocytes were fused to myeloma cells (P3Ag8.653 cell line) to create hybridoma clones. Supernatant from hybridoma clones was tested with binding ELISA as the primary screening assay to identify positive clones binding to BMP9. Supernatant of positive clones identified from primary screening binding assay was then tested in blocking ELISA to identify positive clones that can inhibit the interactions between BMP9 and its receptors. Four different recombinant BMP9 receptors were used: human Alk1-Fc (R&D system, 370-AL-100); human BMPRII-Fc (R&D system, 811-BR-100); human ActRIIA-Fc (R&D system, 340-R2-100); human ActRIIB-Fc (R&D system).
[0518]Two clones were sele...
example 3
n of Anti-BMP9 Antibodies by Phage Display Technology
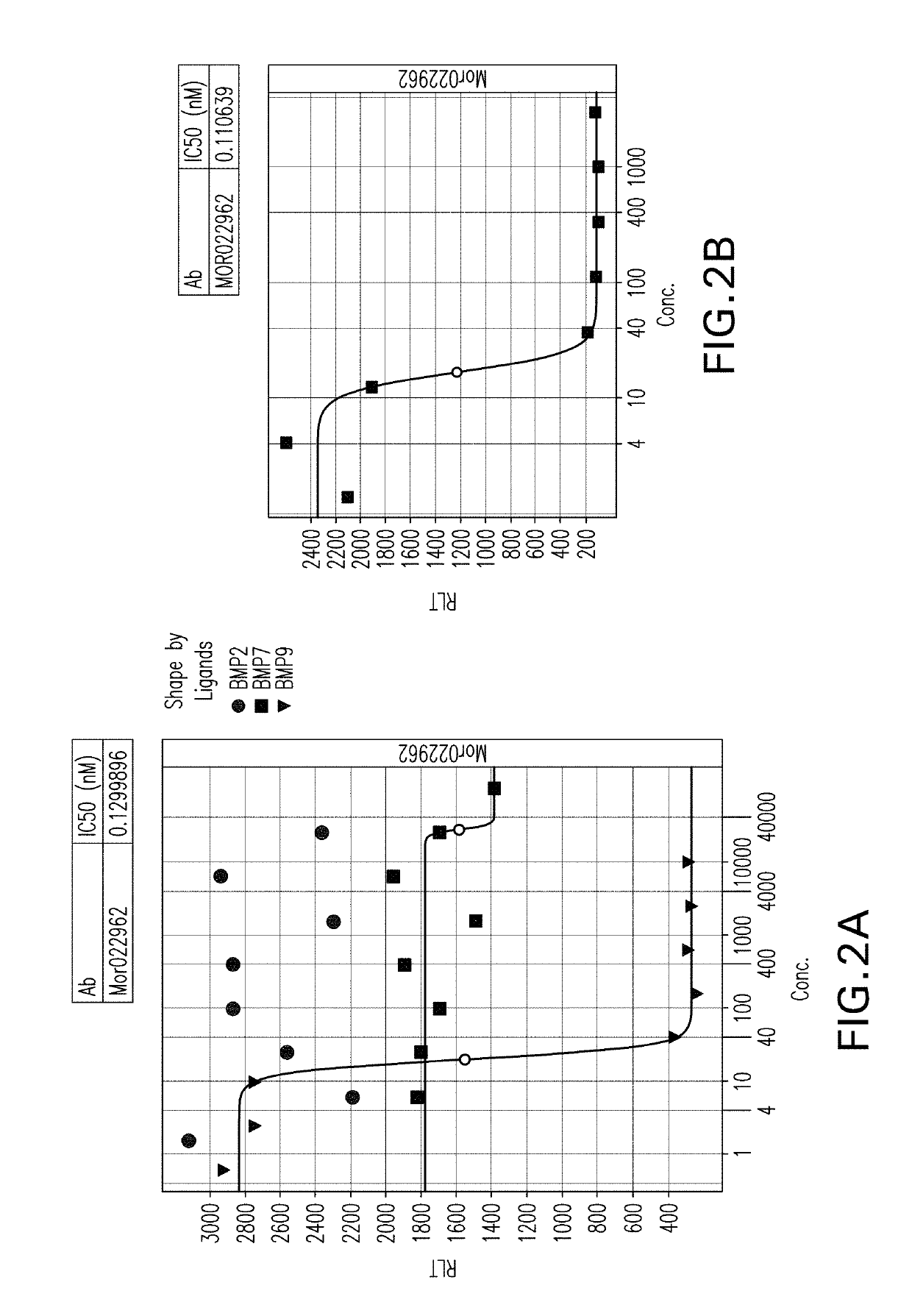
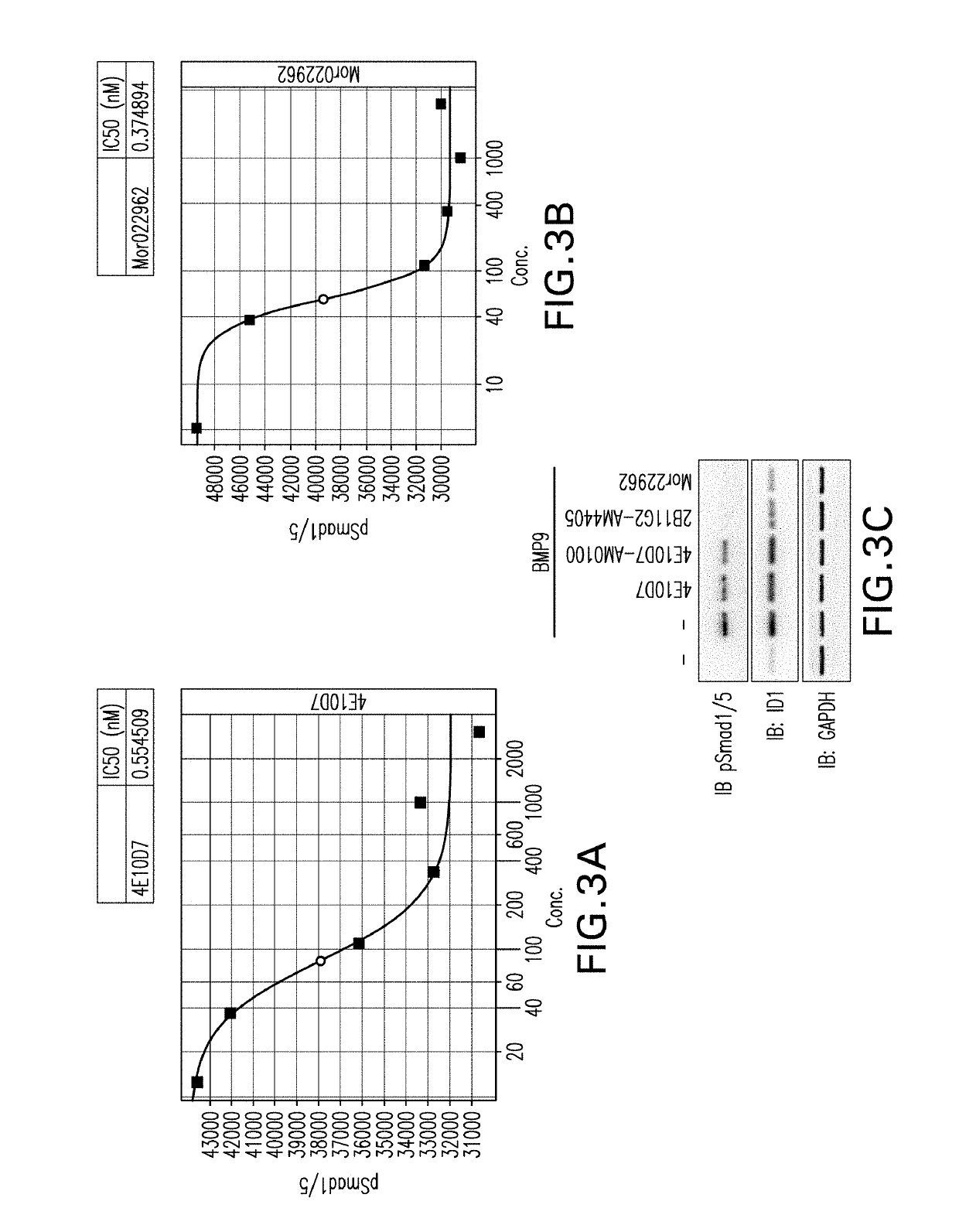
[0536]In parallel with efforts to identify anti-BMP9 antibodies by mouse hybridoma and humanization procedures, described above, phage display was used to identify fully human anti-BMP9 antibodies. Briefly, for the selection of antibodies recognizing human BMP9, multiple panning strategies were employed. Therapeutic antibodies against human BMP9 protein were generated by selection of clones having high binding affinities, using as the source of antibody variant proteins a commercially available phage display library, the MorphoSys HuCAL PLATINUM® library. The phagemid library is based on the HuCAL® concept (Knappik et al., (2000) J Mol Biol 296:57-86) and employs the CysDisplay® technology for displaying the Fab on the phage surface (WO01 / 05950 to Lohning) In order to increase antibody binding affinity whilst maintaining library diversity the second round output of both solution and solid phase pannings were entered into the RapMA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com