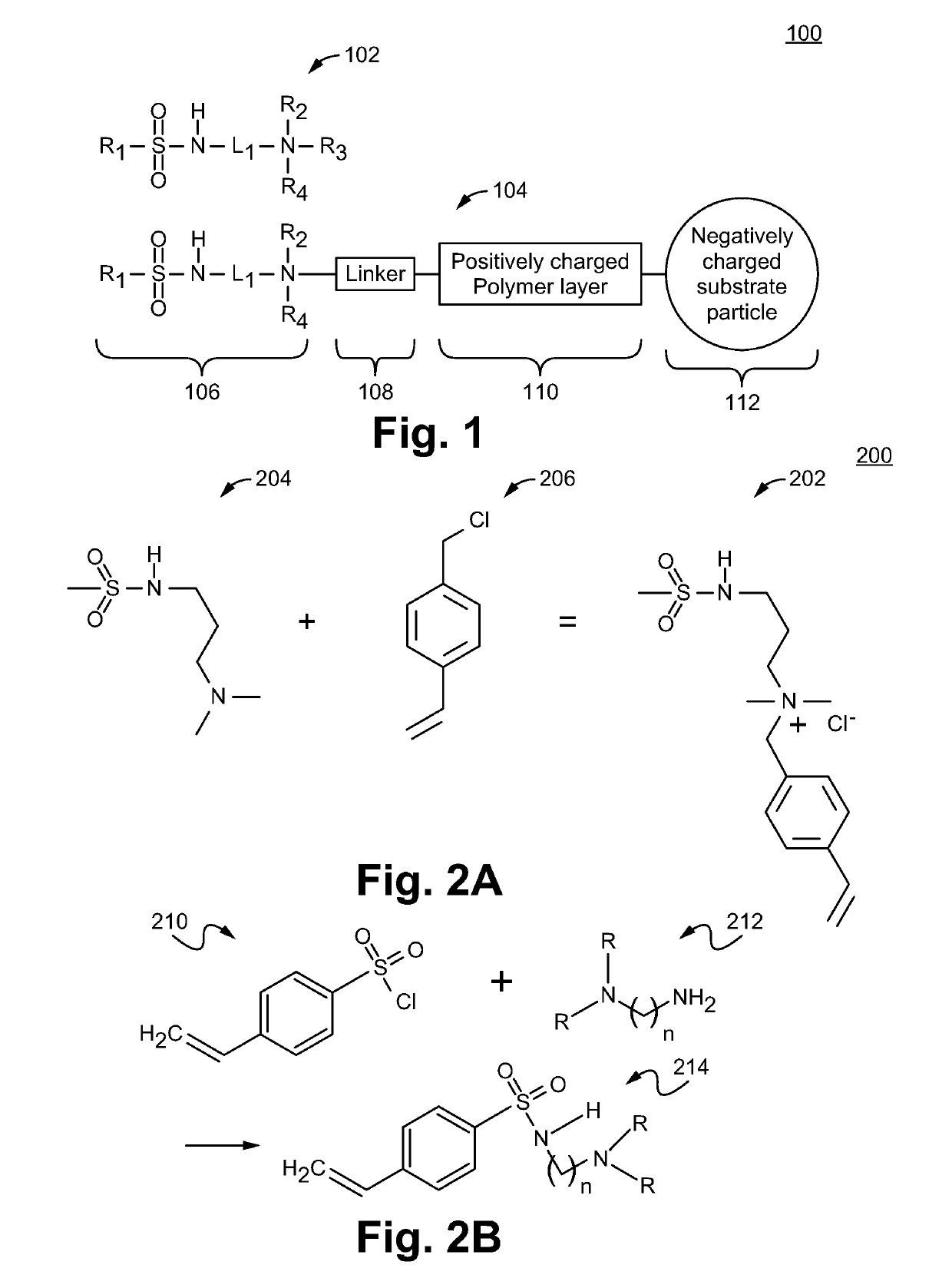
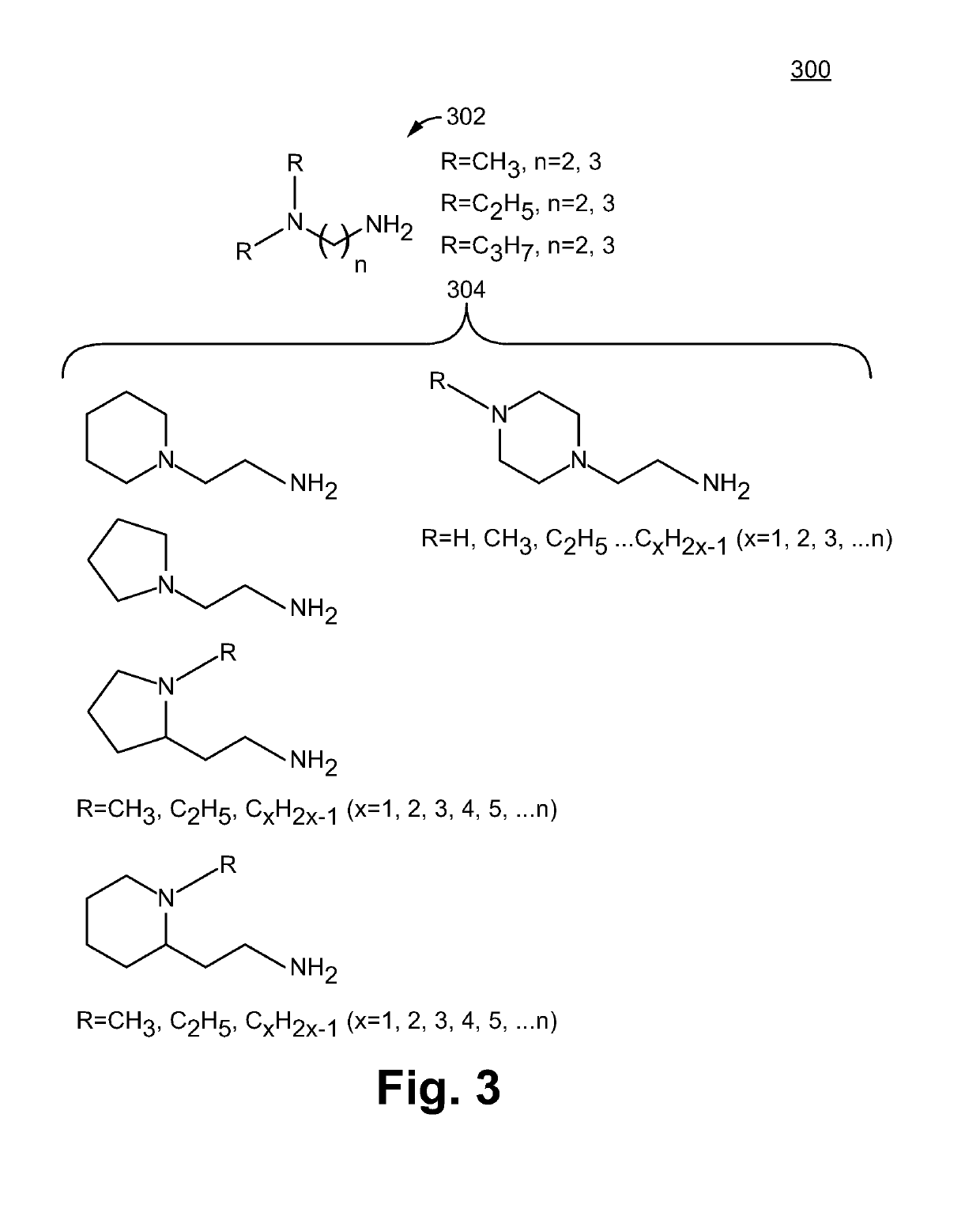
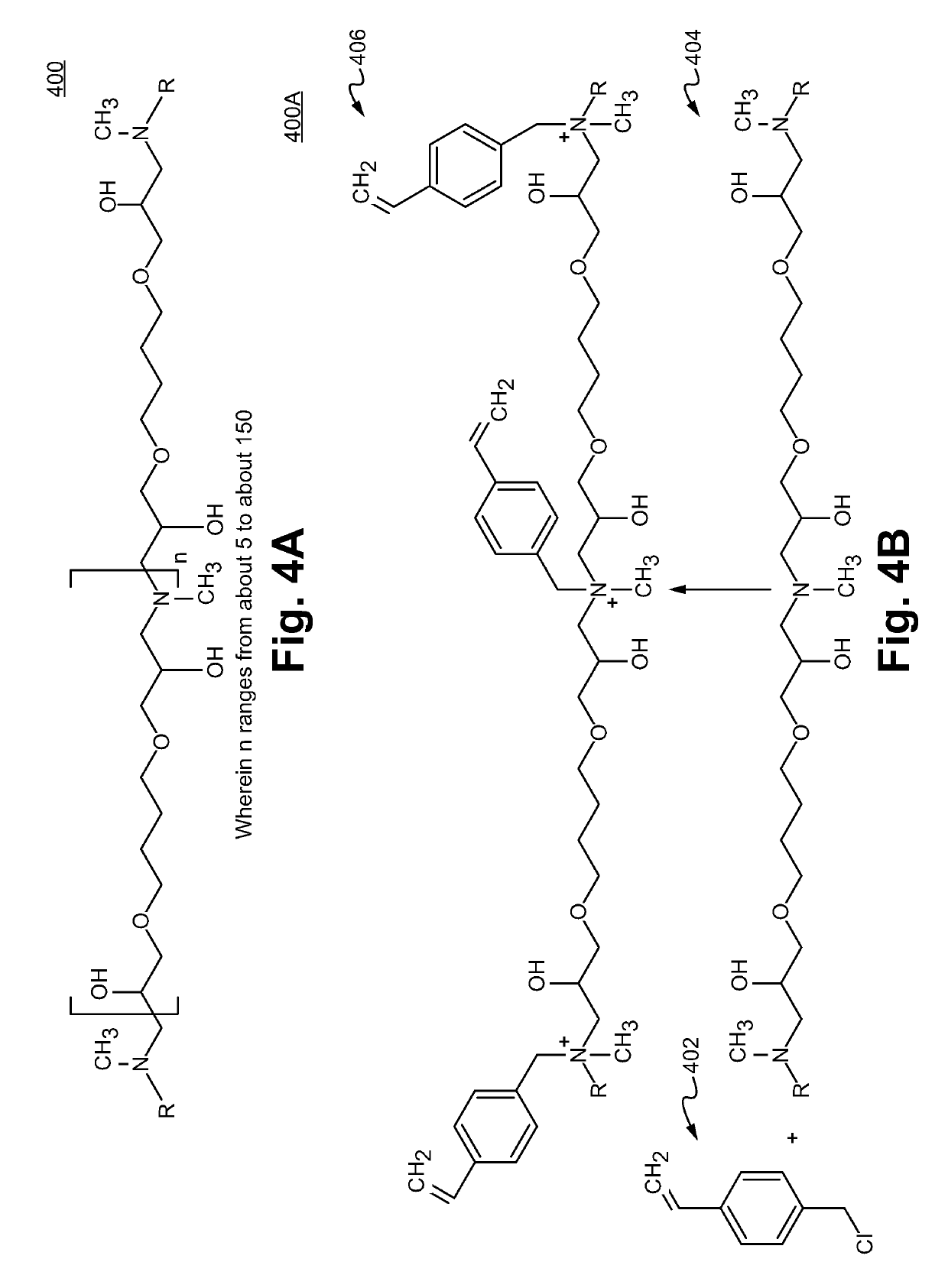
Sulfonamide based anion exchange resins
a technology of anion exchange resin and sulfonamide, which is applied in the field of chromatographic sample separation, can solve the problem that the sample containing the analyte(s) of interest can have a relatively high ionic strength, and achieve the effect of minimizing the retention of trivalent species
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sulfonation of SMP Resin
[0072]25 g of SMP resin was dispersed in 125 g of glacial acetic acid. 500 g of concentrated sulfuric acid was slowly added to the dispersion. Next, it was thoroughly mixed and sonicated in a water bath at room temperature for 60 minutes. The reaction mixture was poured over ˜1000 g of ice. Once the reaction mixture equilibrated to room temperature, the reaction mixture was filtered, and washed with DI water (deionized water) until the washing showed a pH close to neutral. The resin was isolated for further functionalization.
example 2
Procedure For Making Sulfonated Resin with Positively Charged Polymer that Includes Grafted VBC
[0073]20 g of sulfonated SMP resin was packed into a 9×250 mm column. A combination of 72% 1,4-butanediol diglycidyl ether (10 wt % solution in DI water) and 28% methyl amine (4 wt % solution in DI water) was pumped through the column while being maintained at 65° C., at a flow rate of 0.5 ml / min for 60 min. The column was then unpacked and the resin was slurried in 100 mL of DI water with sonication for 30 seconds with a probe sonicator and sieved through a 38 μm sieve and filtered. Next, the resin was then dispersed in 100 mL of methanol and filtered. It was then rinsed with 2 aliquots of 50 mL of methanol. The resin was then stirred gently in 100 ml of a 5% solution of vinylbenzylchloride (VBC) in methanol for 3 to 4 hrs at 60° C. The mixture was filtered and the resin washed with 4 aliquots of 50 ml of MeOH (methanol) and 3 aliquots of 50 ml of DI water. The sulfonated resin with a pos...
example 3
Procedure For Grafting Sulfonamide / Quaternary Amine / VBC Monomer
[0074]1.7 g of N,N-Dimethyl-N-vinylbenzyl-aminopropyl methylsulfonamide monomer 202 was dissolved in 10 g of DI water. 5 g of the resin from Example 2 was then dispersed in this solution and 0.2 g of initiator (e.g., 2,2′-Azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride, Wako VA-044) is added and thoroughly mixed. The mixture was then tumbled at 52° C. for 12-16 hrs. The reaction mixture was then diluted to 100 ml with DI water, filtered and washed with 1) DI water, 2) Acetone, 3) DI water, 4) 0.5M NaOH, 5) DI water and finally 0.5M Na2CO3. The resin was then isolated for testing.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com