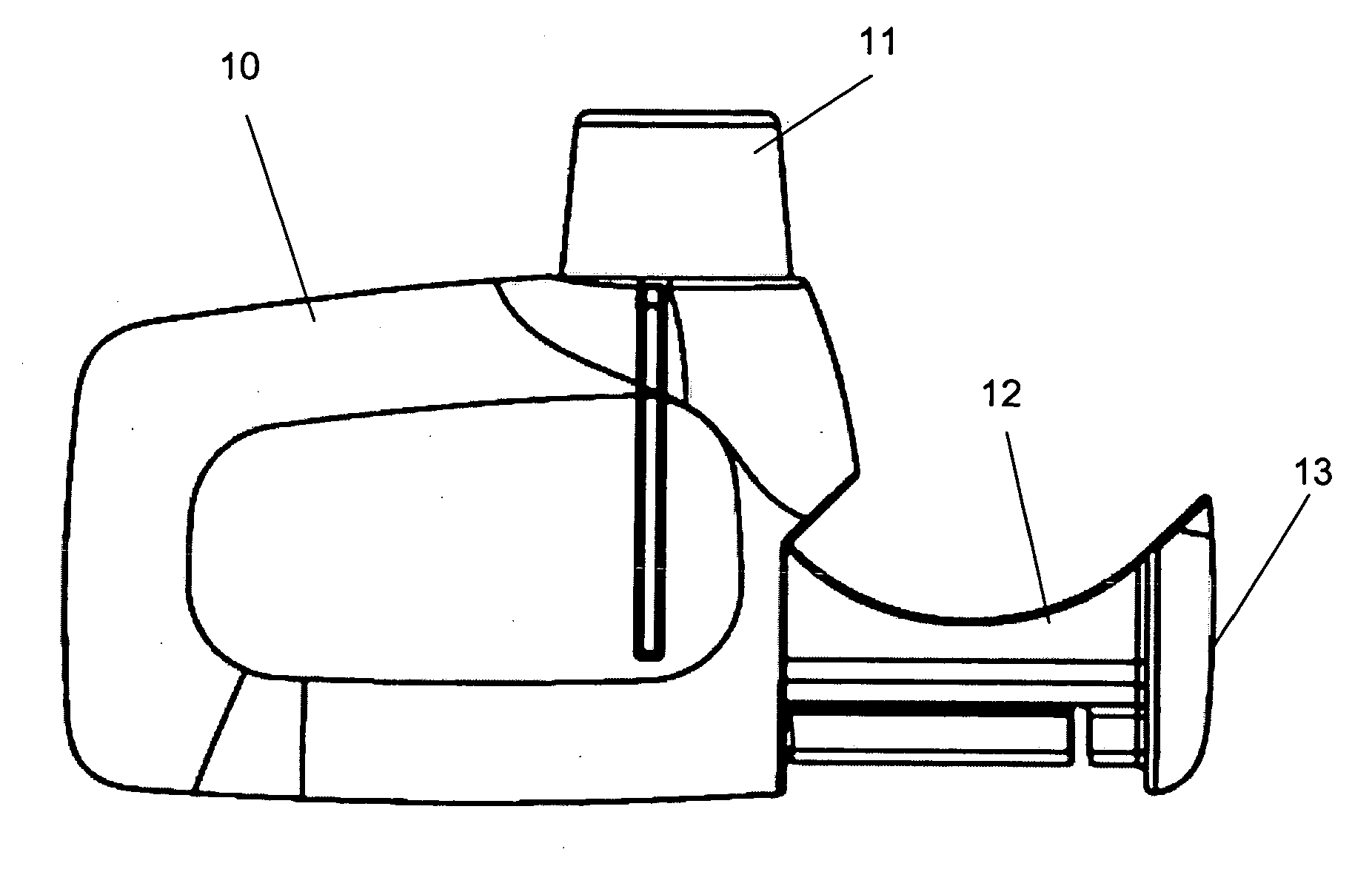
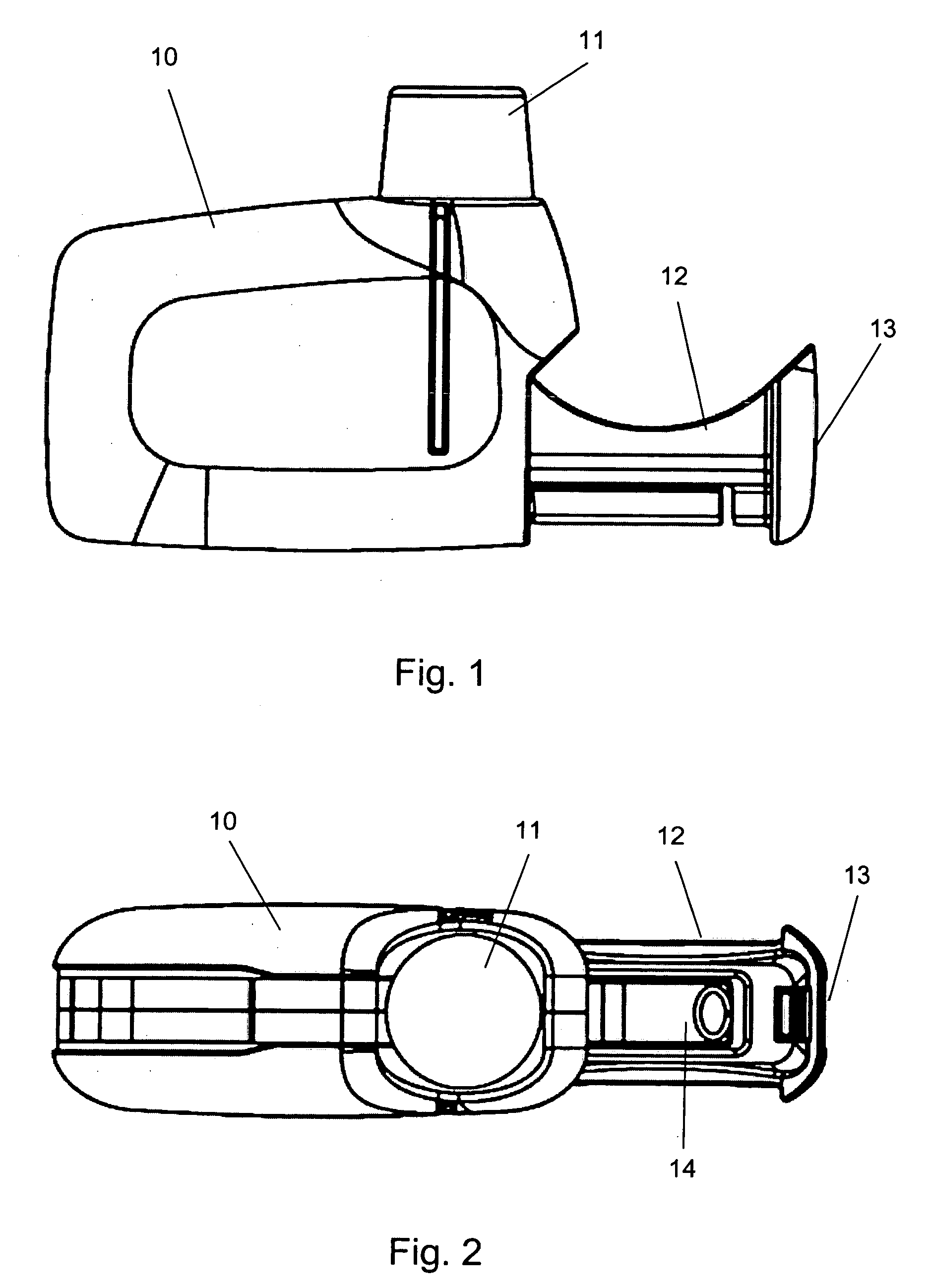
Inhaler
a technology of inhaler and powder, which is applied in the field of dry powder inhaler, can solve the problems of uncontrollable shearing force, unacceptable, and unsuitable parts of the dose, and achieve the effect of reducing retention and high air speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The present invention discloses a novel type of manually operated, dry powder inhaler device (DPI) adapted for accepting a dose container enclosing a metered dry powder dose. The disclosed inhaler device is preferably a single dose inhaler relying on the power of a user for delivering dry powder doses of medicaments. The inhaler device can have a movable slide, which is intended to be loaded with a replaceable dose container by a user. The user inserts the dose container into the inhaler body, for example by pushing the slide into the body by hand force. When the dose in the dose container has been delivered by the DPI, the slide is brought out of the inhaler body, at any convenient point in time. The user then can remove the spent container and can, if desired, push the now empty slide again into the inhaler body or, for example, load a new dose container into the slide in preparation of a new dose delivery. In this manner a new container with a new dose may be administered ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com