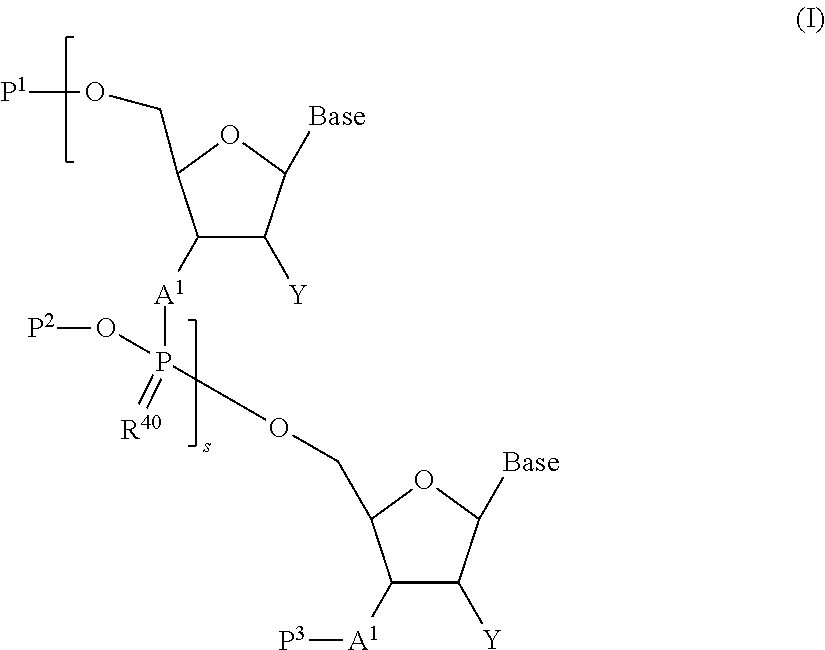
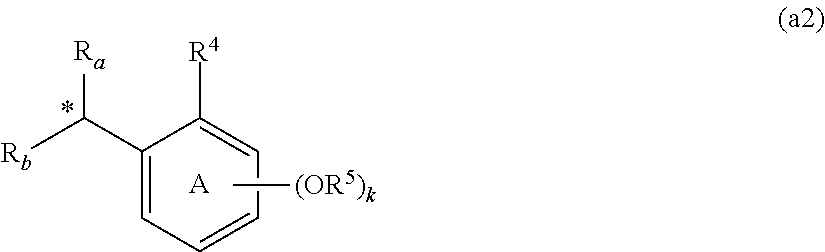Oligonucleotide manufacturing method
a production method and technology of oligonucleotides, applied in the field of oligonucleotide production methods, can solve the problems of inferior preservation stability of 2-cyanoethyl-n,n-diisopropylchlorophosphoramidite, safety problems in deprotection use of hydrazine, etc., and achieve the effect of producing more stably and efficiently
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 4
t of Decyanoethylaed Specimen Using DBU
[0202]DMTr-d[TGiBuCCO-TOP]-TBDMS (0.0043 g, 0.00179 mmol) obtained in the same manner as in Example 2 was dissolved in tetrahydrofuran (0.15 mL). 1,8-Diazabicyclo[5.4.0]undec-7-ene (hereinafter DBU) (2.0 μL, 0.0133 mmol) was added and mixed. Decyanoethylation was performed at 23° C. for 5 min. To the reaction mixture was added acetonitrile (1.0 mL) and the mixture was centrifuged at 10,000G, 4° C. for 5 min. The supernatant was removed, the remaining was dispersed in acetonitrile (1.0 mL) and similarly centrifuged again. Whole precipitate was dried in vacuo to give 0.0017 g in weight. When 31P{1H}NMR(CDCl3) of the resultant product was measured, all peaks appeared in 56.5-56.8 ppm, and it was confirmed that a decyanoethylated form shows 31P{1H}NMR signals in a region remarkably different from that of a cyanoethyl-protected form.
example 5
Various Fluoride Ion Sources Using Desilylation
(1) Study Using 3HF-TEA
[0203](DMTr-d[TGiBuCCO-TOP]-TBDMS (0.0239 g, 0.00998 mmol) obtained in the same manner as in Example 2 was dissolved in dichloromethane (0.3 mL) and cooled to 15° C. A solution of 3HF-TEA (29.0 mg, 0.180 mmol) in THF (0.60 mL) was added thereto and they were mixed. Desilylation was performed at 15° C. for 24 hr. The reaction mixture was divided into 4 by about 200 μL, acetonitrile (1.0 mL) was added to each, and the mixture was centrifuged at 10,000G, 4° C. for 5 min. The supernatant was removed, the remaining was dispersed in acetonitrile (1.0 mL) and similarly centrifuged again. Whole precipitate was dried in vacuo to give 0.0230 g in weight. When 31P{1H}NMR(CDCl3) of the resultant product was measured, all peaks were observed in 65.8-68.1 ppm corresponding to a cyanoethyl-protected form, and a signal in 60 ppm or below corresponding to a decyanoethylated form was not observed. In the measurement by LC-TOF MS, c...
example 21
Activation and Phosphitylation Using 4,5-dichloroimidazole
(1) Determination of pKa by Titration of 4,5-DICHLOROIMIDAZOLE
[0321]4,5-Dichloroimidazole (0.2396 g, 1.749 mmol) was dissolved in ion exchange water deaerated by boiling to 50.0 mL (0.0350 M). This solution (20 mL) was titrated at 25° C. with 0.10 M NaOH.
[0322]By non-log linearization plot of titration results (Benet, L. Z.; Goyan, J. E. J. Pharm. Sci. 1967, 56, 665-680.), pKa of dichloroimidazole in water at 25° C. was determined to be 9.09.
(2) Diamidite Activation and Phosphitylation Using 4,5-dichloroimidazole
[0323]2-Cyanoethyl-N,N,N′,N′-tetraisopropylphosphordiamidite (0.120 g, 0.40 mmol) was dissolved in dry toluene:cyclohexane=1:1 (v / v) solvent (1.5 mL). Thereto was added 4,5-dichloroimidazole (0.138 g, 1.00 mmol) and the mixture was stirred at under dry argon for 17 hr to prepare a solution containing a phosphitylating agent. The total amount of this solution was filtered through a 0.45 μm membrane filter. N-methylmorp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com