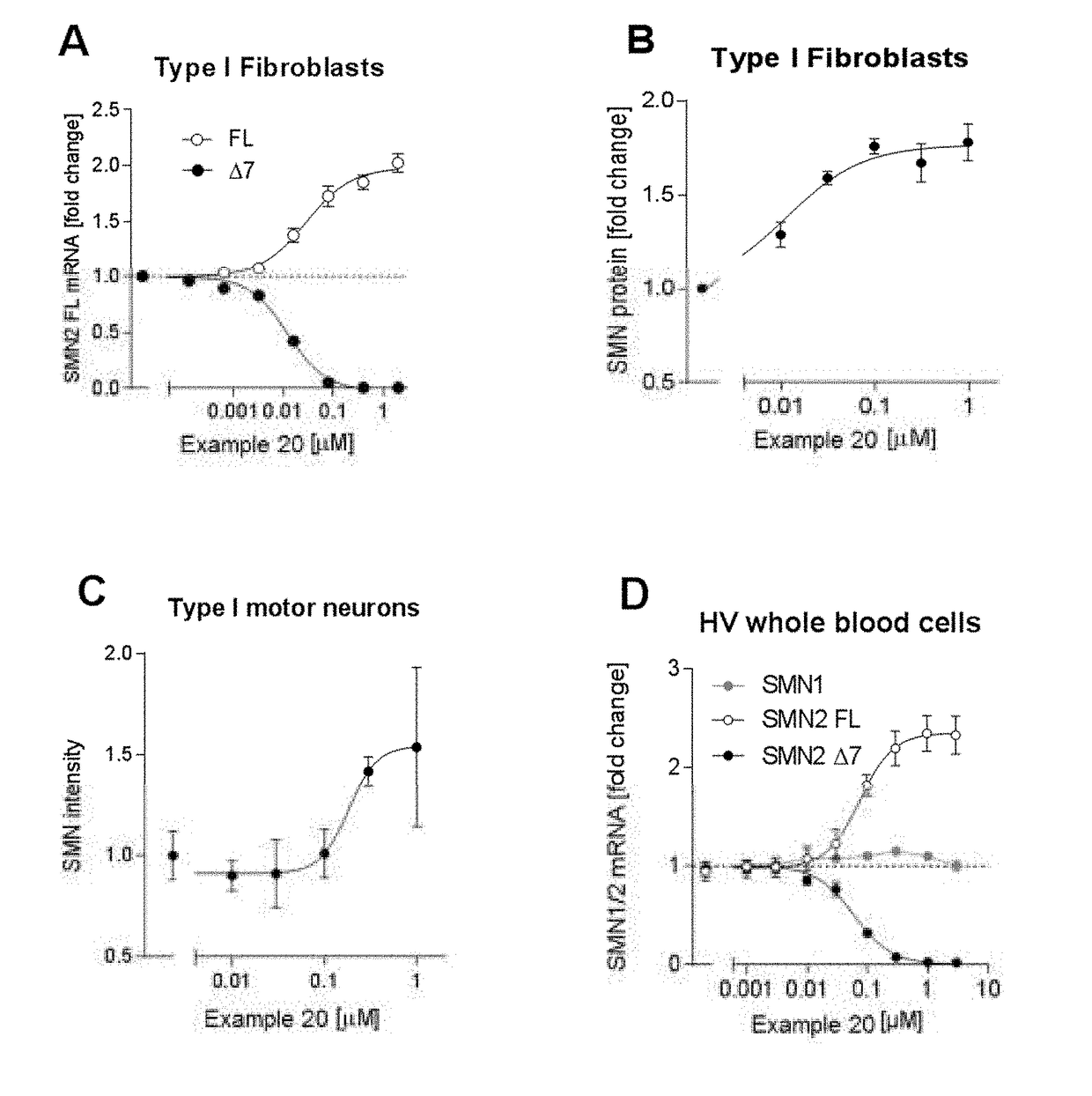
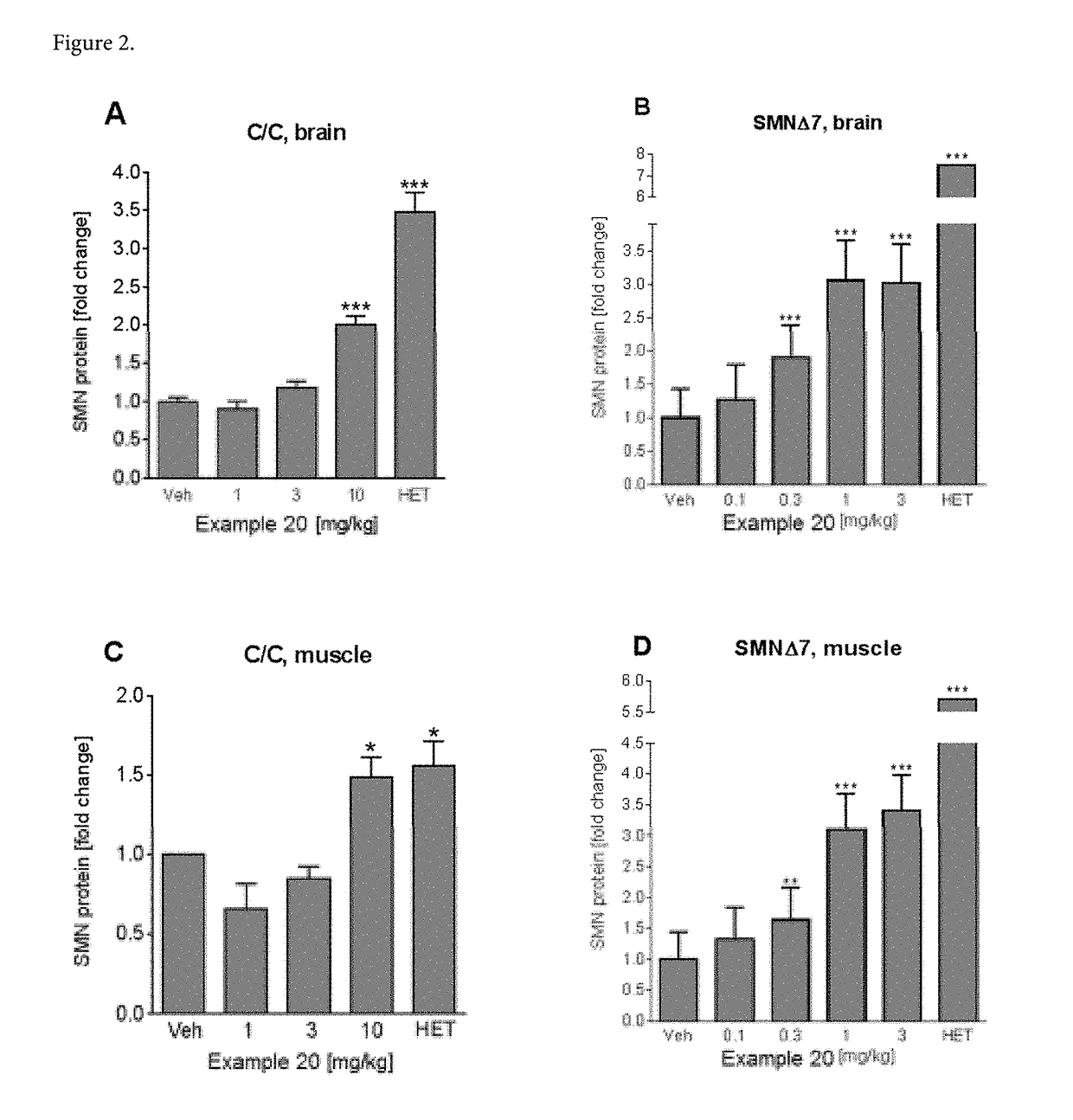
Compositions for treating spinal muscular atrophy
a technology of spinal muscular atrophy and compositions, applied in the direction of muscular disorder, drug compositions, dispersed delivery, etc., can solve the problems of muscle weakness, poor muscle tone, weak cry,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
2-(2-methylimidazo[1,2-b]pyridazin-6-yl)-7-(4-methylpiperazin-1-yl)pyrido[1,2-a]pyrimidin-4-one
[0550]
[0551]In a sealed tube, 7-fluoro-2-(2-methylimidazo[1,2-b]pyridazin-6-yl)-4H-pyrido[1,2-a]pyrimidin-4-one (Intermediate 1; 35 mg, 0.119 mmol) and 1-methylpiperazine (47.5 mg, 0.474 mmol, 4 eq.) were stirred in DMSO (1 mL) at 120° C. overnight. LC-MS showed total convertion. The solvent was removed under high vacuum. The crude product was purified by column chromatography (SiO2, CH2Cl2 / MeOH=95 / 5 to 9 / 1) to afford the title product (25 mg, 56%) as a light yellow solid. MS m / z 376.3 [M+H+].
example 2
7-[(8aR)-3,4,6,7,8,8a-hexahydro-1H-pyrrolo[1,2-a]pyrazin-2-yl]-2-(2-methylimidazo[1,2-b]pyridazin-6-yl)pyrido[1,2-a]pyrimidin-4-one
[0552]
[0553]In a sealed tube, 7-fluoro-2-(2-methylimidazo[1,2-b]pyridazin-6-yl)-4H-pyrido[1,2-a]pyrimidin-4-one (Intermediate 1; 125 mg, 0.426 mmol) and (R)-octahydropyrrolo-[1,2-a]pyrazine (160 mg, 1.27 mmol, 3 eq.) were stirred in DMSO (5 mL) at 125° C. overnight. The solvent was removed under high vacuum. The residue was taken up in CH2Cl2 and washed with an aqueous saturated solution of NaHCO3. The organic layer was separated and dried over Na2SO4 and concentrated in vacuo. The crude was purified by column chromatography (SiO2, CH2Cl2 / MeOH=98 / 2 to 95 / 5) to afford the title product (65 mg, 38%) as a light yellow solid. MS m / z 402.5 [M+H+].
example 3
7-[(8aS)-3,4,6,7,8,8a-hexahydro-1H-pyrrolo[1,2-a]pyrazin-2-yl]-2-(2,8-dimethylimidazo[1,2-b]pyridazin-6-yl)pyrido[1,2-a]pyrimidin-4-one
[0554]
[0555]In a sealed tube, 2-(2,8-dimethylimidazo[1,2-b]pyridazin-6-yl)-7-fluoro-pyrido[1,2-a]pyrimidin-4-one (Intermediate 2; 200 mg, 0.647 mmol) and (S)-octahydropyrrolo-[1,2-a]pyrazine (286 mg, 2.26 mmol, 3.5 eq.) were stirred in DMSO (5 mL) at 125° C. overnight. The solvent was removed under high vacuum. The residue was taken up in CH2Cl2 and washed with an aqueous saturated solution of NaHCO3. The organic layer was separated and dried over Na2SO4 and concentrated in vacuo. The crude was purified by column chromatography (SiO2, CH2Cl2 / MeOH=98 / 2 to 95 / 5) to afford the title product (115 mg, 43%) as a light yellow solid. MS m / z 416.3 [M+H+].
PUM
| Property | Measurement | Unit |
|---|---|---|
| pKa | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com