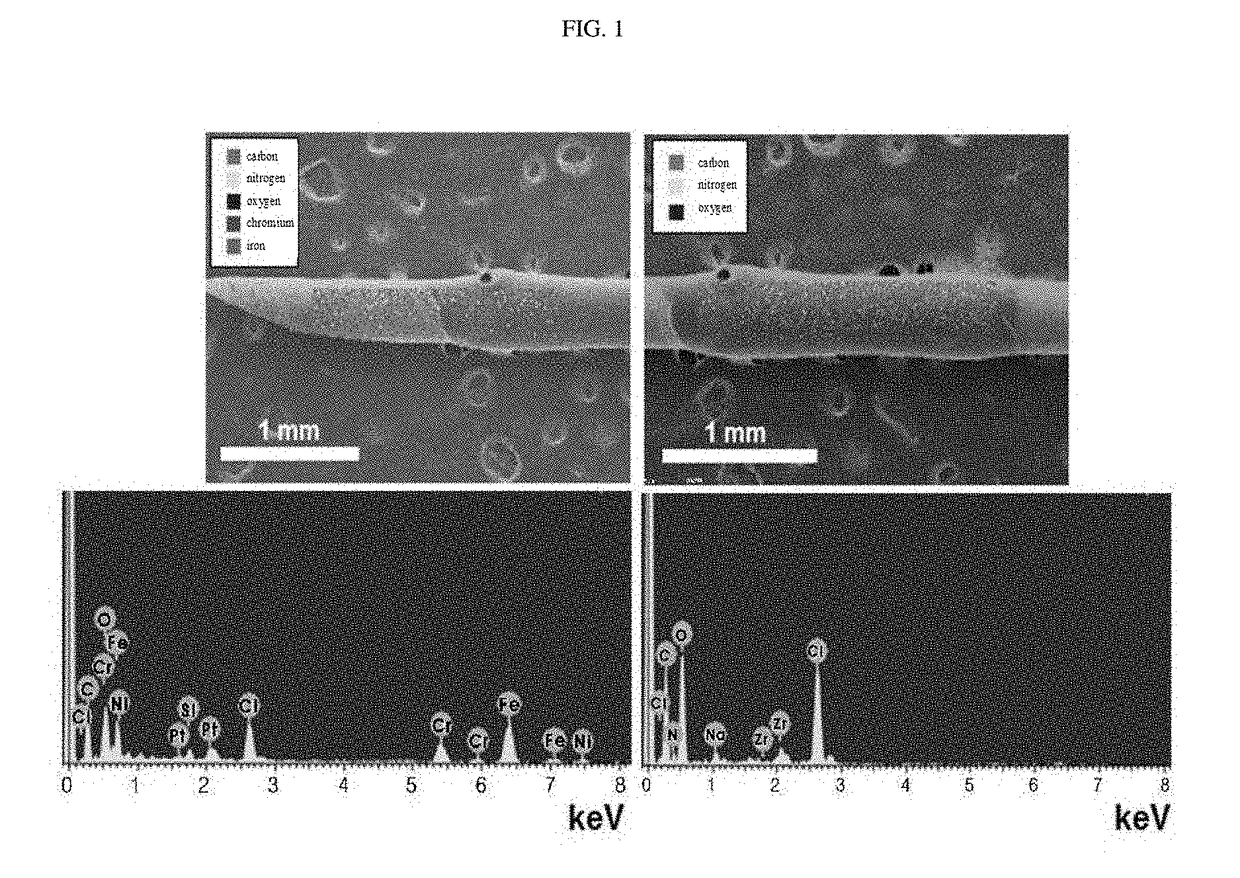
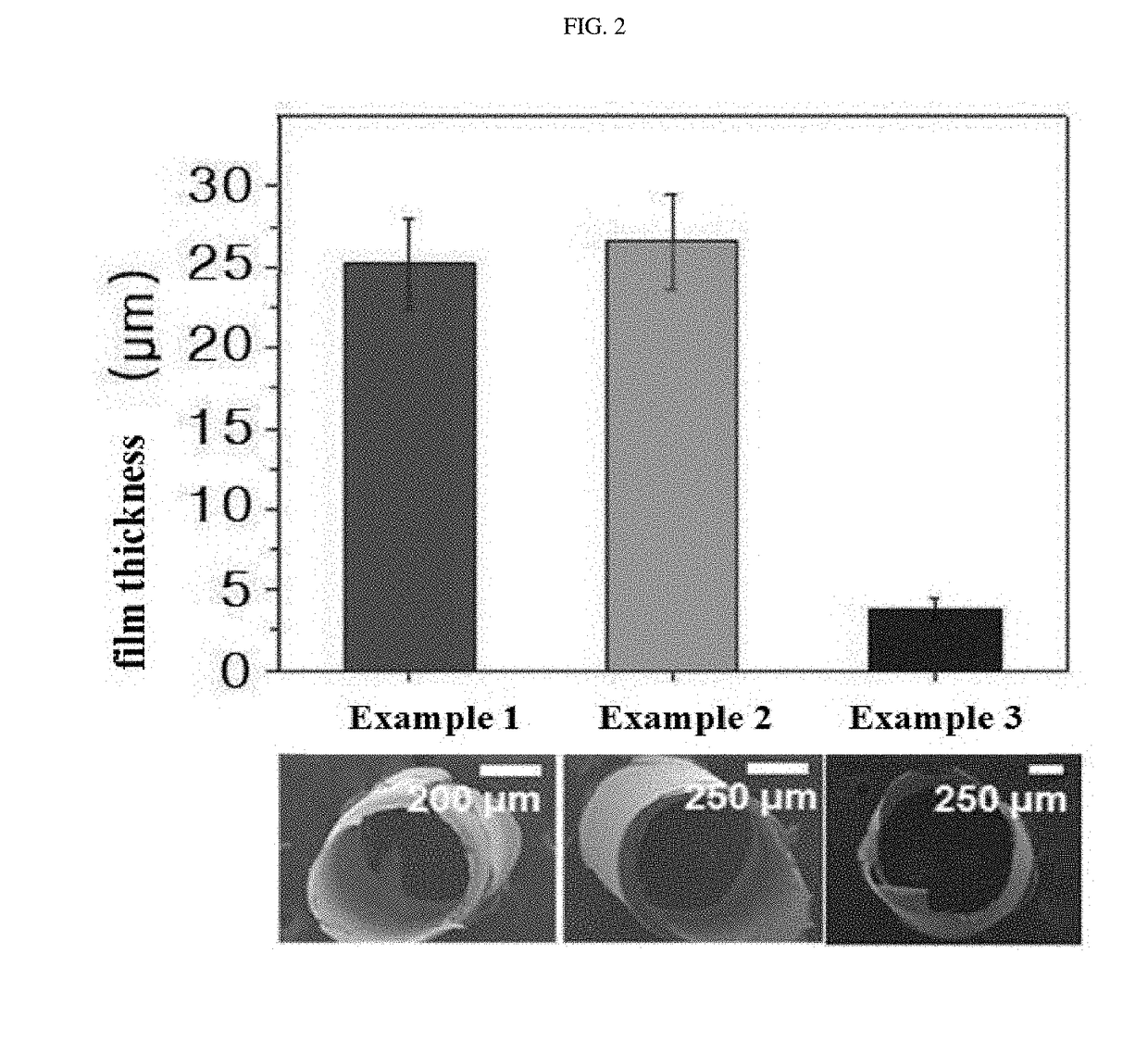
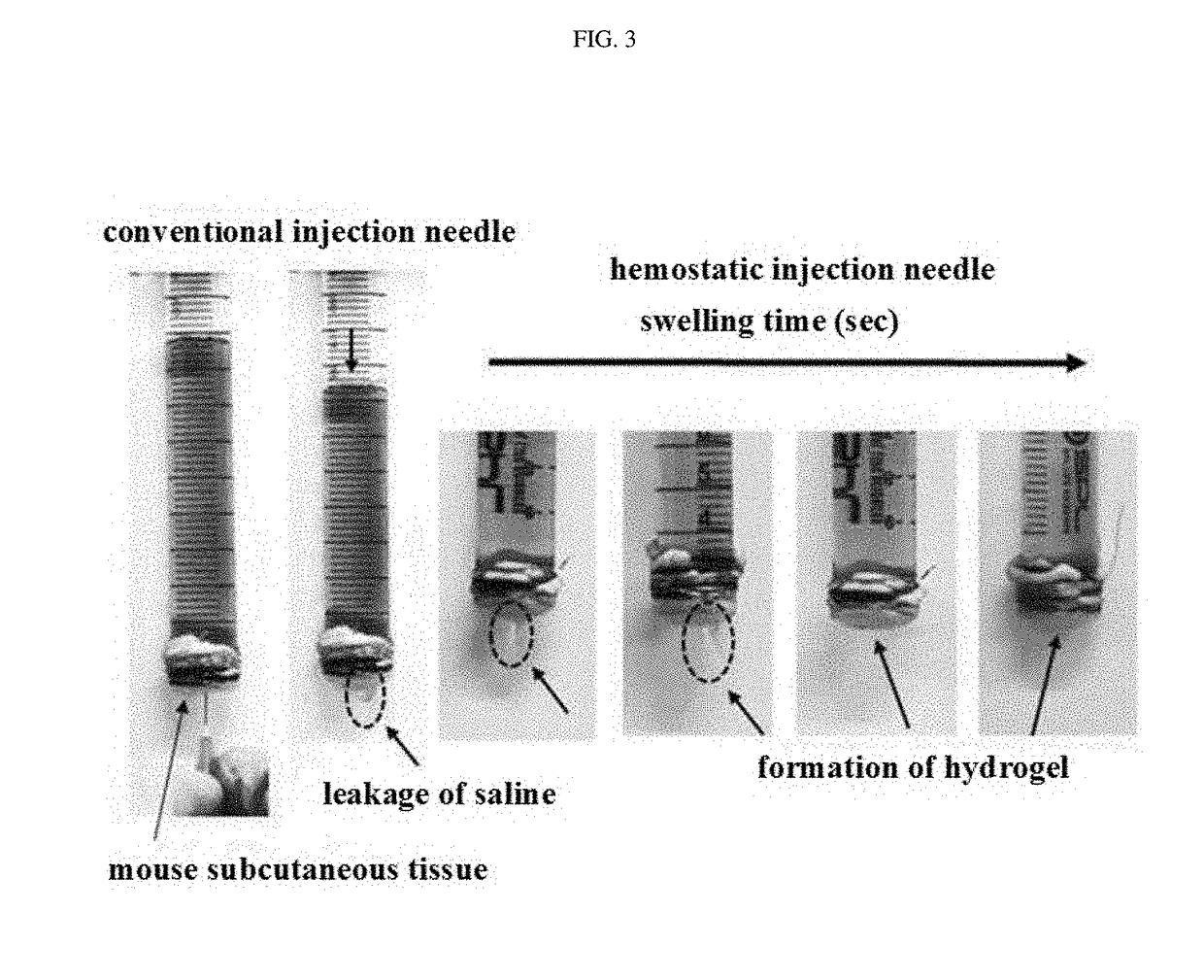
Hemostatic injection needle coated with crosslinked chitosan having catechol group and oxidized catechol group
a technology of chitosan and injection needle, which is applied in the direction of coating, surgery, pharmaceutical delivery mechanism, etc., can solve the problems of significant low solubility in neutral solutions, the development of materials having excellent adhesion to tissue, and the inability to meet all the adhesive strength requirements, so as to prevent bleeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
Preparation of Chitosan Introduced with Catechol
[0072]
[0073]3 g of about 30% acetylated chitosan (chitosan 70 / 100, model: 24204, manufactured by Heppe Medical Chitosan) was dissolved in 292 mL of HCl solution (pH=2) for 6 hours. The chitosan solution was adjusted to a pH of 5.5 by slowly adding 8 mL of 0.5 N NaOH solution thereto. The prepared 1% chitosan solution was stabilized for 12 hours.
[0074]To the prepared chitosan solution, 2.37 g of 3-(3,4-dihydroxyphenyl)propanoic acid was added. Then, as a reactant for forming an amide bond (—CONH—) between the amine (—NH2) group of the chitosan and the carboxyl group (—COOH) of 3-(3,4-dihydroxyphenyl)propanoic acid, 2.02 g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) was dissolved in 50 mL of ethanol and added to the chitosan solution. Next, the solution was adjusted to a pH of 4.5, and then allowed to react for 1 hour. In this process, a catechol-functionalized chitosan was prepared.
[0075]To remove unreacted 3-(3,4-dihydroxyph...
example 1
Manufacture 1 of Hemostatic Injection needle (26 G)
[0076]1.5 mL of the catechol-functionalized chitosan prepared in Preparation Example 1 was dissolved in 100 μL of triple-distilled water, and then stored at 4° C. for 3 days to induce partial oxidation and cross-linking of the catechol group. As a result, a chitosan solution (hereinafter referred to as “chitosan-catechol solution” was prepared in which the catechol group
and the oxidized catechol group
were introduced and partially cross-linked.
[0077]The degree of oxidation of the catechol group in the chitosan-catechol solution was determined by measuring the absorbance at a wavelength of 500 nm by UV-Vis spectrometry. When a cross-link between the oxidized catechol group and the amine group of the chitosan is formed, the absorbance at a wavelength of 500 nm appears. Thus, based on the absorbance appearing the chitosan-catechol solution was treated with NaIO4 (that is an oxidizing agent that induces oxidation of the chitosan-catechol...
example 2
Manufacture 1 of Hemostatic Injection needle (23 G)
[0079]A hemostatic injection needle was manufactured in the same manner as described in Example 1, except that a 23 G-thick injection needle was used instead of the 26 G-thick injection needle and that the chitosan-catechol solution was used in an amount of 9 μL instead of 6.5 μL.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com