Alzheimer abeta peptide binding polypeptide
a technology of alzheimer's disease and binding polypeptide, which is applied in the field of alzheimer's disease abeta peptide binding polypeptide, can solve the problems of no treatment that significantly alters the course of the disease or efficiently stops its development, and achieves the effects of improving the folding of fusion proteins, increasing expression, and increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Design and Cloning of Affinity Maturation Libraries
Summary
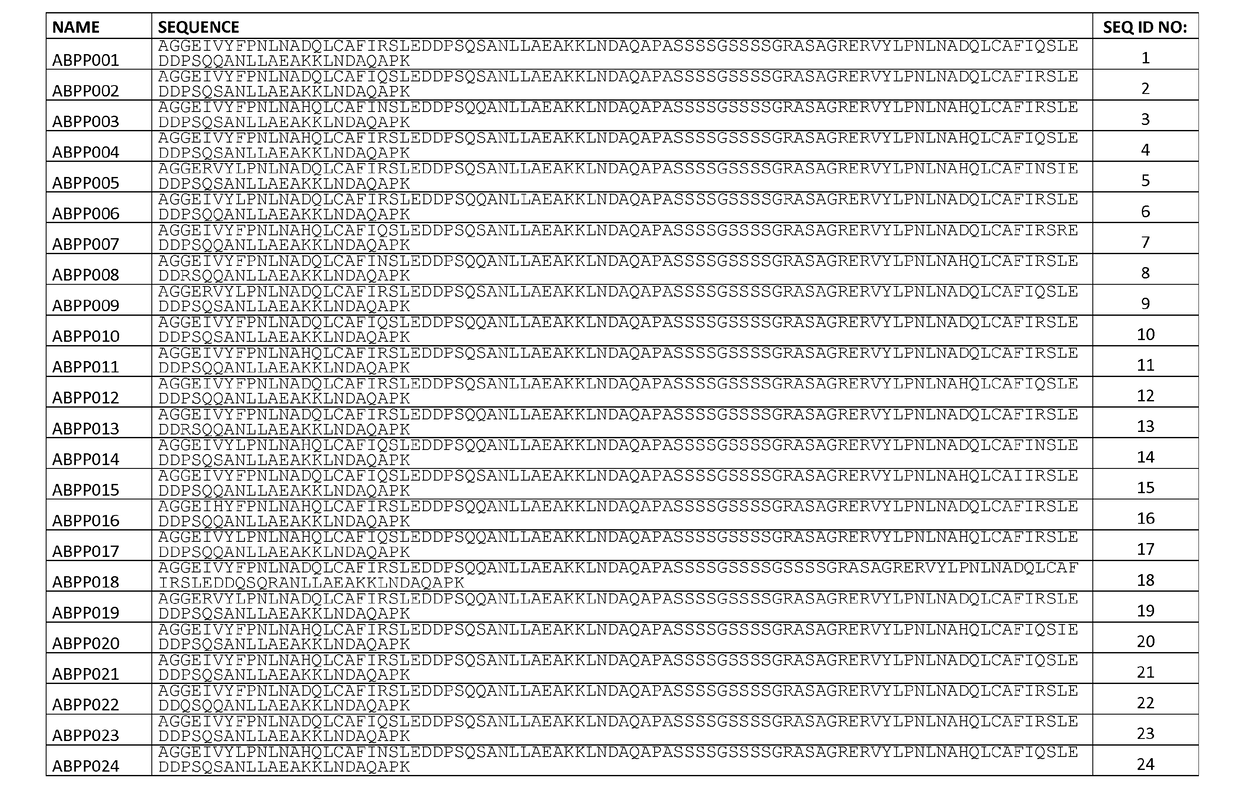
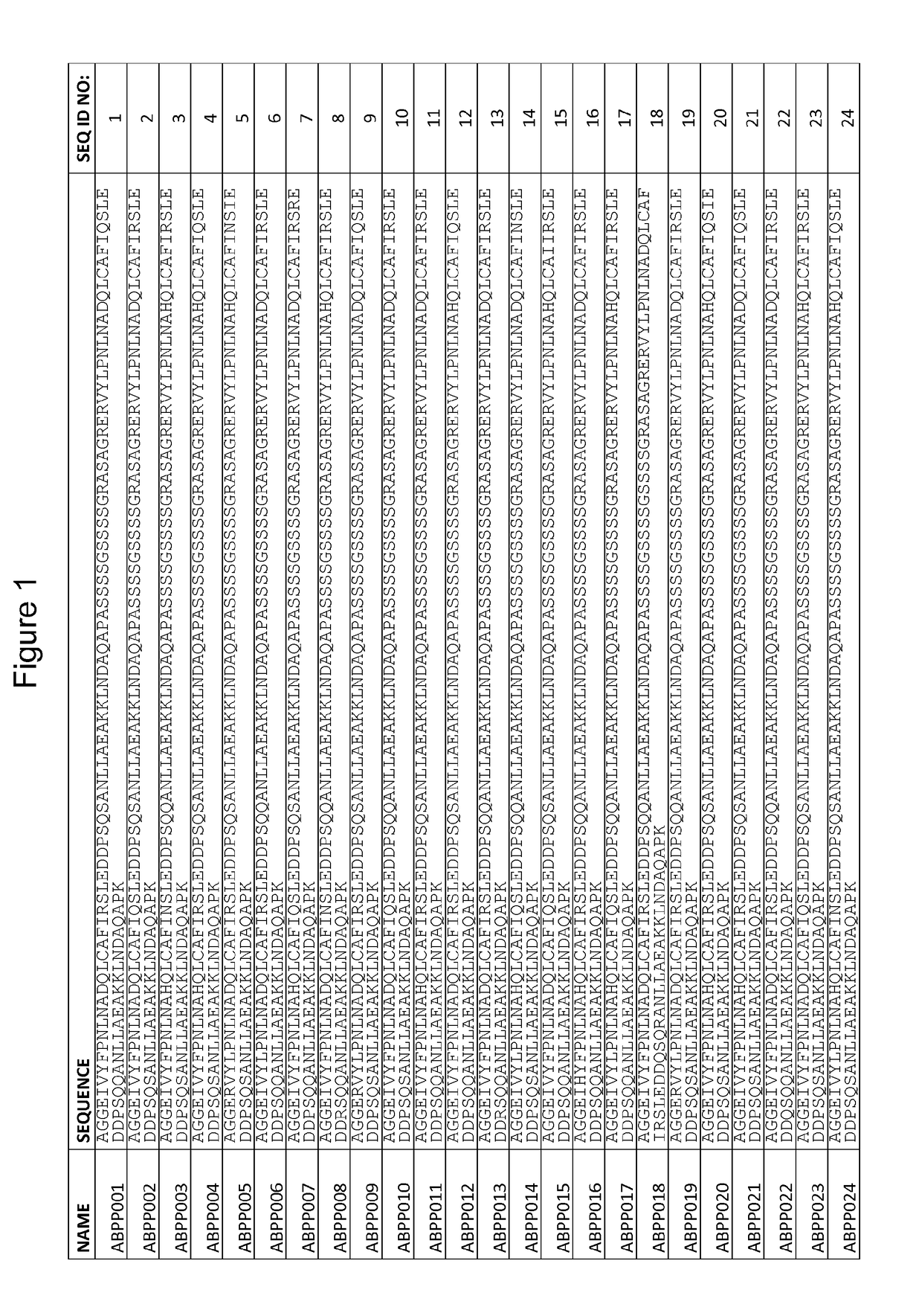
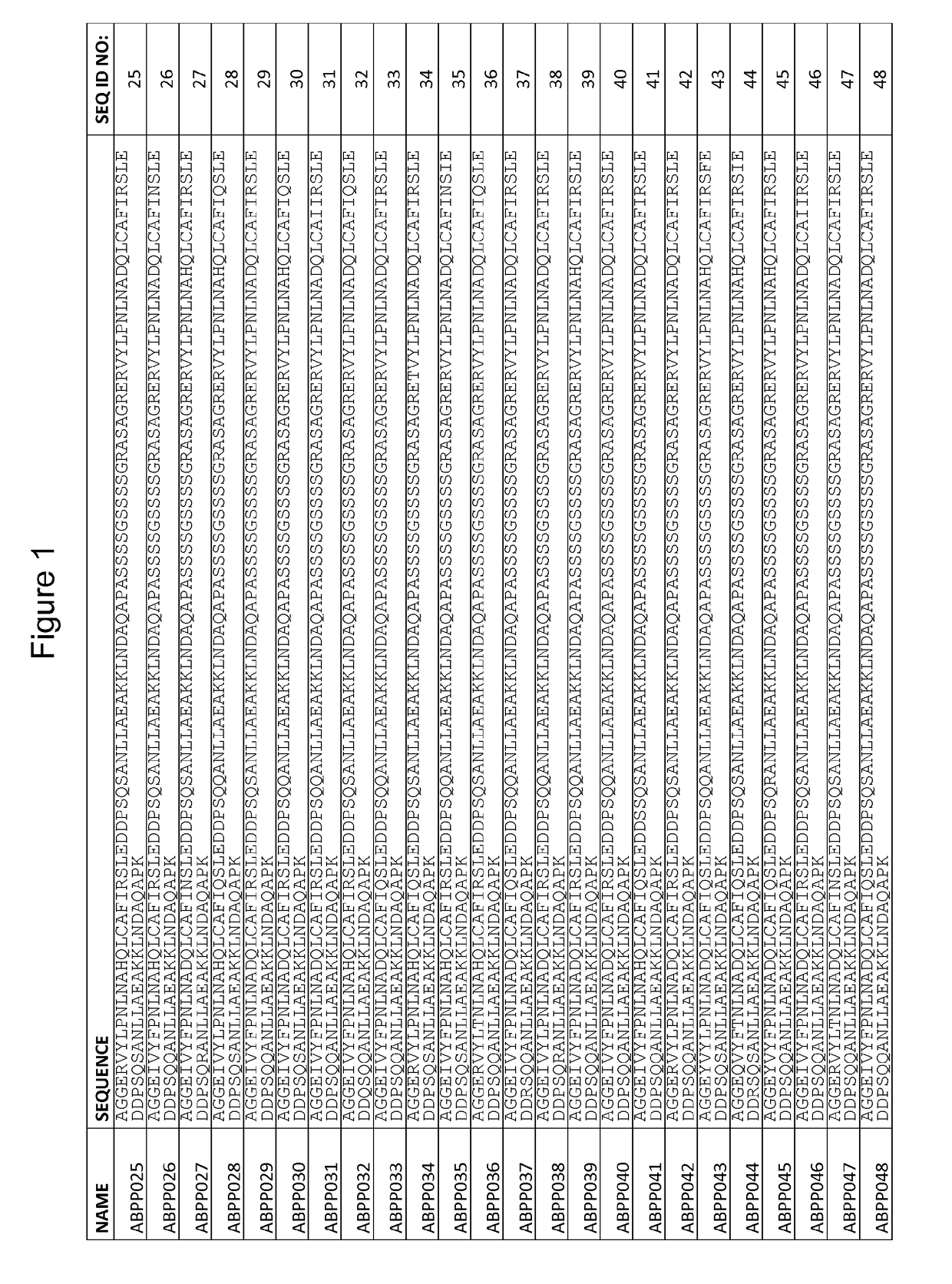
[0238]This Example describes the design and cloning of two affinity maturation libraries, ZASlib and ZSYMlib, based on dimers of the previously identified first generation Aβ peptide binding polypeptide variant ZAβ3 (SEQ ID NO:111).
Materials and Methods
[0239]Two SlonoMax® head-to-tail dimer libraries of double-stranded DNA were purchased from Sloning BioTechnology GmbH (Pucheim, Germany). Library oligonucleotides encoded the truncated helix 1 plus helix 2 and 3 of the first Z variant unit and helix 1 and 2 of the second Z variant unit, making up the first and second moieties of the Aβ peptide binding polypeptides (asymmetric library: 5′-GCG GGT GGG GAG NNN NNN TAT NNN NNN AAC TTA AAC GCG NNN CAA CTG TGT GCC TTC ATC NNN AGT TTA GAA GAT GAO CCA AGC CAA NNN GCT AAC TTG TTG GCA GAA GCT AAA AAG CTA AAT GAT GCT CAG GCG CCG GCG AGC AGC AGC AGC GGG AGC AGC AGC AGC GGG CGC GCG AGT GCG GGT CGC GAG NNN GTT TAT TTA NNN AAC TTA AAC GCG NN...
example 2
Flow Cytometric Sorting of Affinity Maturation Libraries and Sequencing of Isolated Polypeptides
Summary
[0244]This Example describes the cloning of the affinity maturation libraries of Example 1 into a vector for staphylococcal display, and subsequent sorting and selection of Aβ peptide binding polypeptides by flow cytometry utilizing increased stringency conditions in each sorting cycle. Sequencing of isolated variants lead to the identification of 51 unique variants from the asymmetric library (SEQ ID NO:1-51) and 55 unique variants from the symmetric library (SEQ ID NO:52-106).
Materials and Methods
[0245]At least ten times the library size of either library was inoculated to tryptic soy broth supplemented with yeast extract (TSB+Y; Merck, Darmstadt, Germany) and 20 μg / ml chloramphenicol, and grown overnight at 37° C. and 150 rpm. The following day, cells were harvested by centrifugation (6000 rpm, 6 min, 4° C.) and washed in phosphate-buffered saline supplemented with 0.1% Pluronic...
example 3
On-Cell Screening for Aβ Binding
Summary
[0249]In this Example, the affinity of 37 isolated polypeptide variants for Aβ peptide was analyzed by flow cytometry.
Materials and Methods
[0250]37 clones (ABPP001, 005, 009, 013, 014, 016, 018, 020, 021, 025, 026, 028, 033, 035, 037, 040, 042, 048, 049 and 050, corresponding to SEQ ID NO:1, 5, 9, 13, 14, 16, 18, 20, 21, 25, 26, 28, 33, 35, 37, 40, 42, 48, 49 and 50, respectively; and ABPP053, 054, 059, 060, 061, 062, 070, 075, 078, 084, 089, 090, 095, 096, 097, 100 and 104, corresponding to SEQ ID NO:53, 54, 59, 60, 61, 62, 70, 75, 78, 84, 89, 90, 95, 96, 97, 100 and 104, respectively), each occurring more than once in the selection results of Example 2, were individually inoculated to TSB+Y with chloramphenicol (10 μg / ml) and grown overnight at 37° C. and 150 rpm. 106 overnight-cultured cells were pelleted by centrifugation and washed in PBSP before resuspension in 1 nM biotinylated Aβ1-40 (AnaSpec). After 45 min incubation at room temperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com