Antibacterial Use of Halogenated Salicylanilides
a technology of halogenated salicylanilides and antibacterial properties, which is applied in the field of halogenated salicylanilides, can solve the problems of ineffective or unsatisfactory treatment of mammal, and achieve the effect of reducing the appearance rate of spontaneous resistant mutants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0147]Experimental tests were conducted to determine the antibacterial activity and the mutation rate conferring resistance for halogenated salicylanilides and reference compounds.
Microorganisms
[0148]Chosen for its relevance regarding bacterial skin infections, the methicillin-resistant S. aureus (MRSA) 01 strain was used as the primary test microorganism. This strain is a community-acquired MRSA clinical isolate of USA 300 type, from a skin abscess.
[0149]Twenty-one other MRSA and methicillin-sensitive S. aureus strains, and 4 Streptococcus pyogenes strains, were also included in the study (Table 1). These covered fusidic acid- and mupirocin-resistant strains, these two types of resistance being of clinical relevance.
[0150]Strains were conserved in Luria Bertani (LB) Broth (S. aureus) or Brain Heart Infusion (BHI) (S. pyogenes) supplemented with glycerol 15% (v / v) at −80° C., and reactivated by isolation on LB (S. aureus) or BHI (S. pyogenes) agar plates. Strains were cultivated in ...
experiment 1
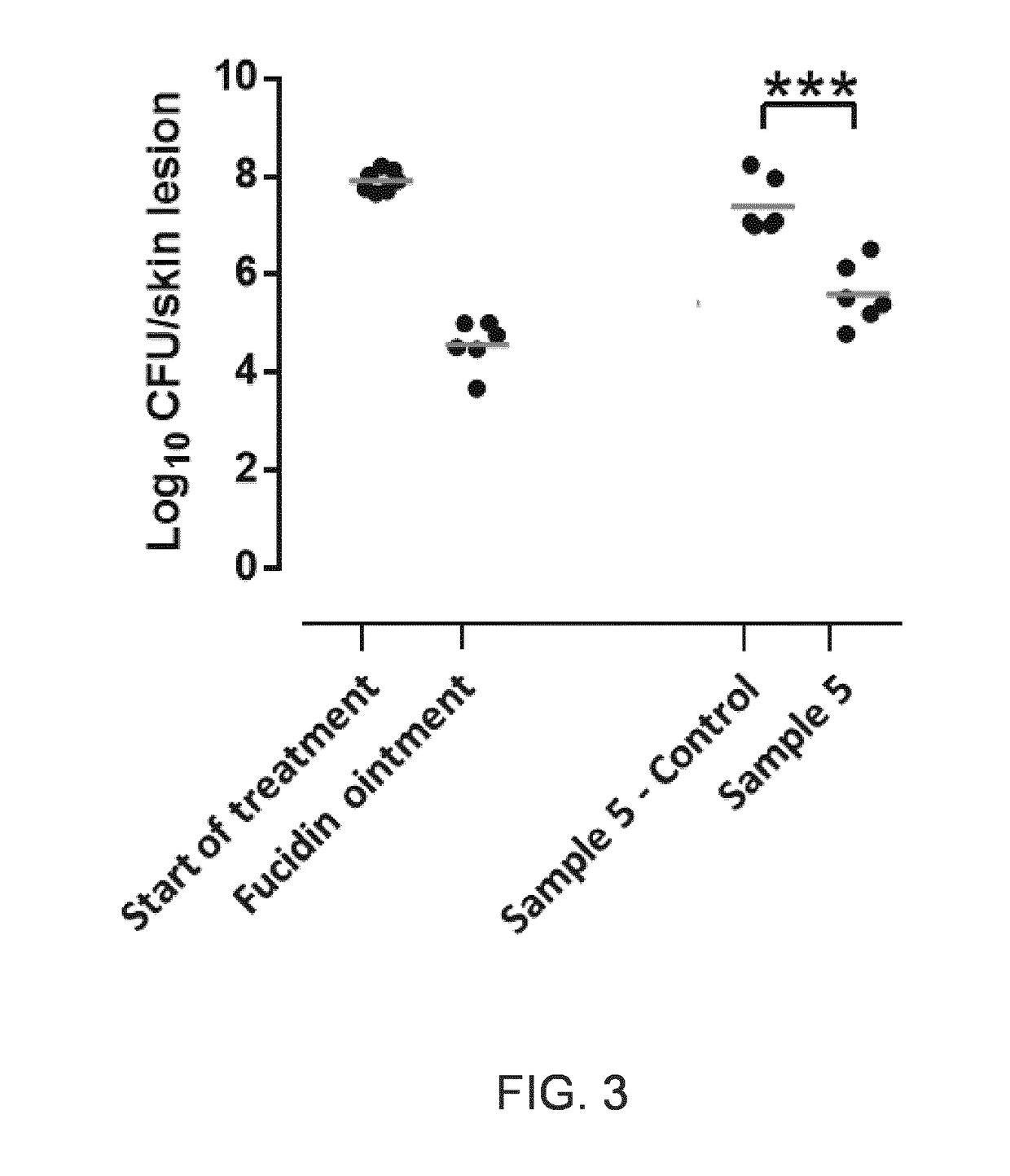
[0167]Sample 5.—Niclosamide (N) 5% modified basis creme with higher lipid content—Lipocreme—according to the description in Danske Laegemiddelsstandarder (DLS).
[0168]2.25 g niclosamide was mixed with 47.75 g créme prepared according to Danske Laegemiddels-standarder (DLS) (see FIG. 3).
Oil phase:Polysorbate 80 10 gCetostearyl alcohol100 gParaffin oil100 gGlycerol monostearate 40-50120 gWater phase:Methyl parabenzoate 1 gGlycerol 85% 40 gSorbitol 70 gWater Milli-Q724 g
Results and Conclusions
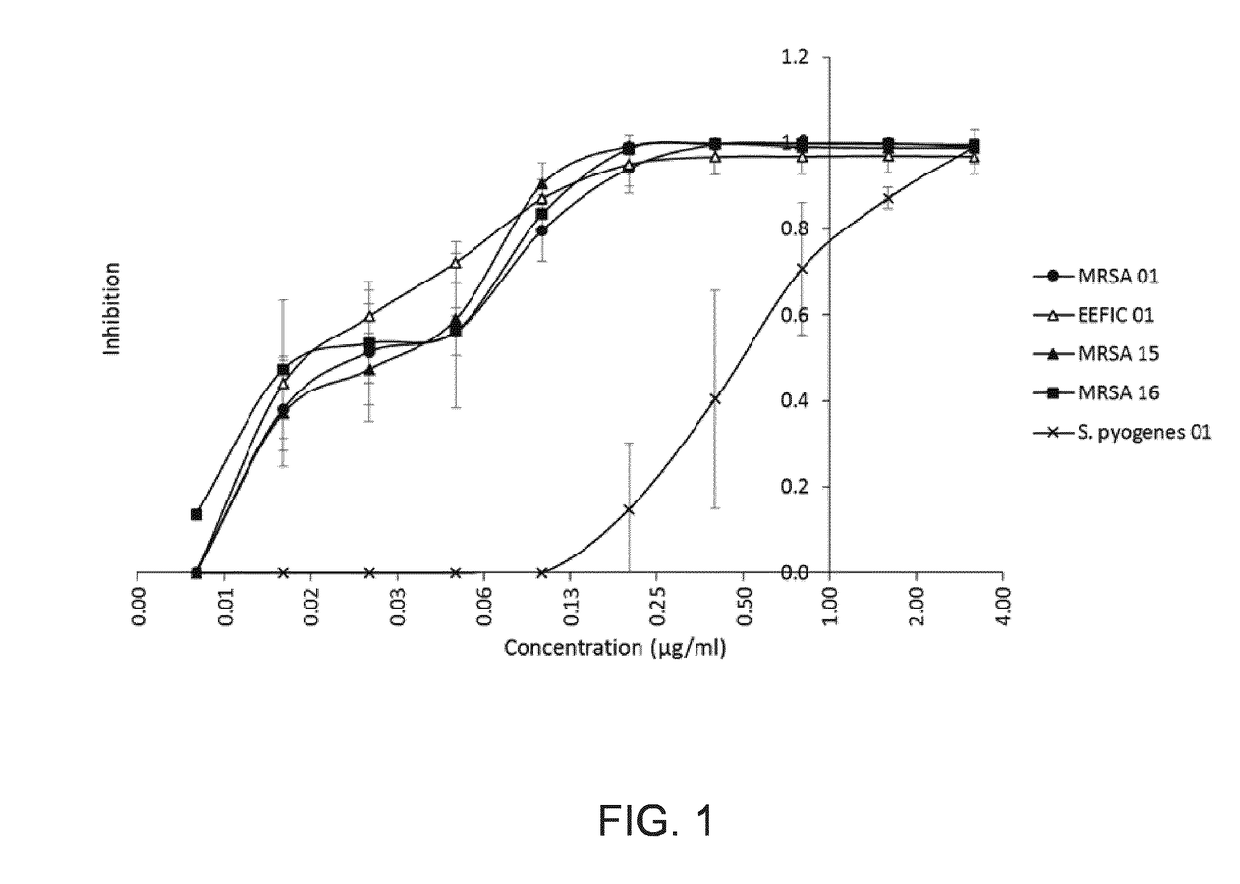
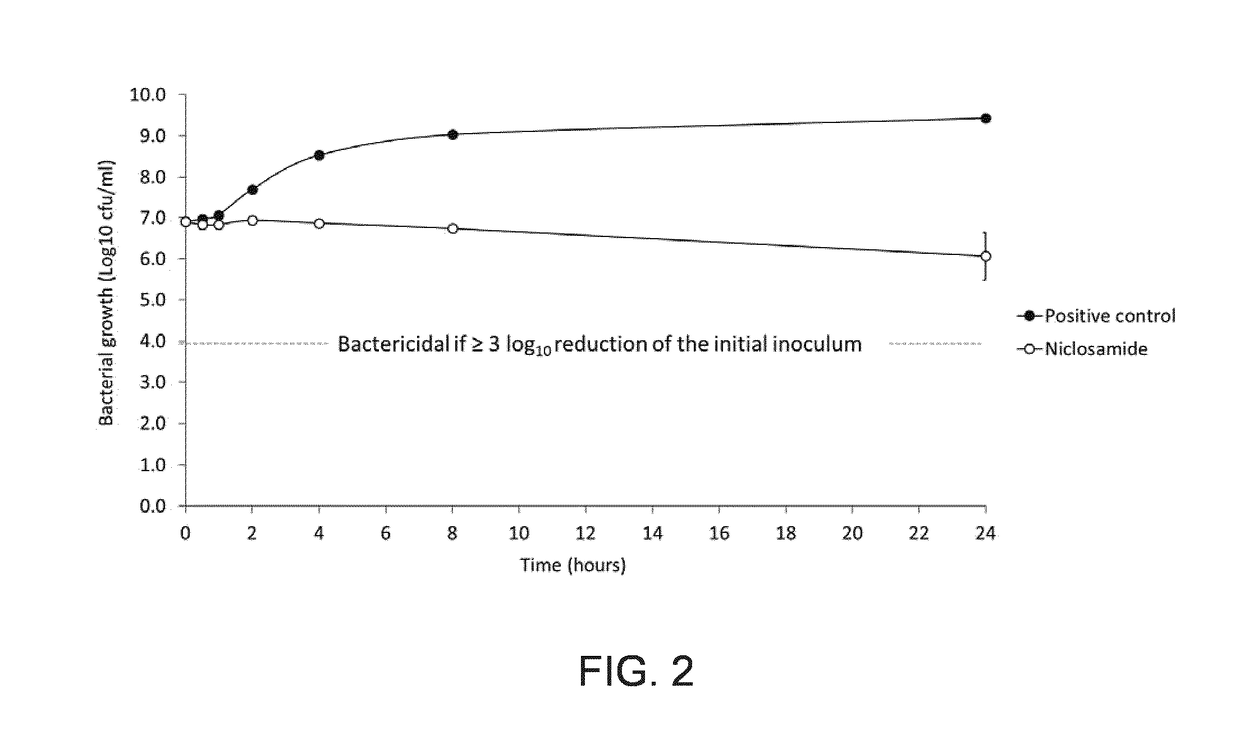
Microbiology: MIC & Kill Curves—FIGS. 1&2 and Tables 2 and 3
[0169]
TABLE 2in-vitro susceptibility of S. aureus clinical isolates and S. aureusATCC 29213 reference strain.MIC (μg / ml)S. aureus strainsNewman0.2MRSA 010.4MRSA 020.2MRSA 03*0.4MRSA 040.4MRSA 050.2MRSA 060.4MRSA 070.2MRSA 08*0.4MRSA 090.2MRSA 100.4MRSA 110.1EEFIC 01*0.4EEFIC 02*0.2MRSA 12*0.4MRSA 13*0.4MSSA 01*0.4MSSA 02*0.4MRSA 14*0.4MRSA 15*0.2MRSA 16*0.2MRSA 17*0.2S. pyogenes strains013.2CCUG 255713.2ATCC 196153.2ATCC 123851.6
TABLE 3The...
example 2
[0179]Additional More Extensive Screen of Clinical Isolates Performed with Niclosamide.
Methods
Microorganisms
[0180]Chosen for its relevance regarding bacterial skin infections, the methicillin-resistant S. aureus (MRSA) 01 strain was used as the primary test microorganism. This strain is a community-acquired MRSA clinical isolate of USA 300 type, from a skin abscess.
[0181]Two-hundred-four other MRSA and methicillin-sensitive S. aureus strains, and 4 Streptococcus pyogenes strains, were also included in the study. These covered fusidic acid- and mupirocin-resistant strains, these two types of resistance being of clinical relevance.
[0182]Strains were conserved in Luria Bertani (LB) Broth (S. aureus) or Brain Heart Infusion (BHI) (S. pyogenes) supplemented with glycerol 15% (v / v) at −80° C., and reactivated by isolation on LB (S. aureus) or BHI (S. pyogenes) agar plates. Strains were cultivated in Mueller Hinton (MH) Broth—cation adjusted (S. aureus) or BHI (S. pyogenes). All strains we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
| Frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com