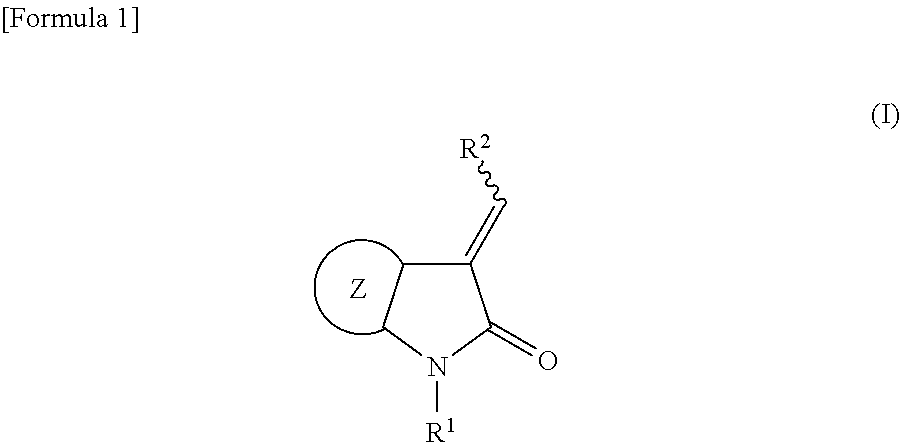
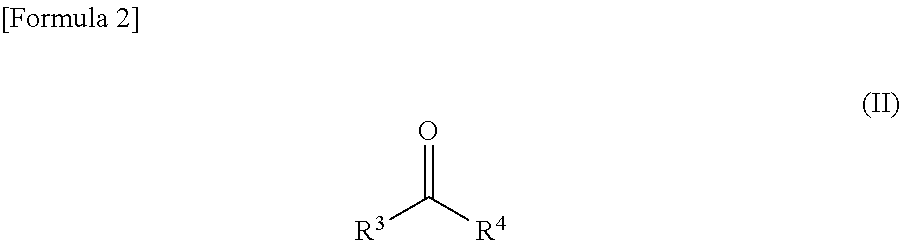
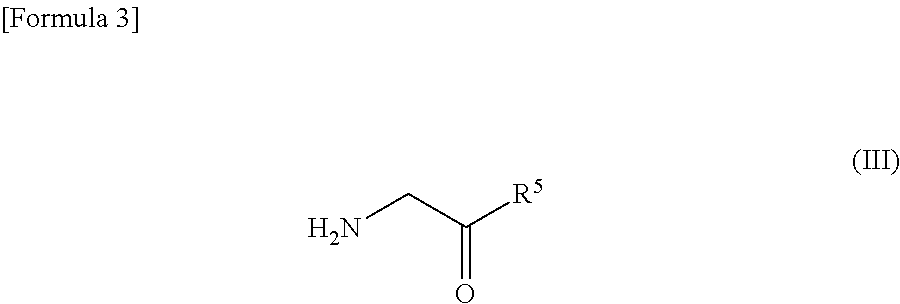
Method for producing spirooxindole derivative
a technology of spirooxindole and derivative, which is applied in the direction of organic compound/hydride/coordination complex catalyst, bulk chemical production, physical/chemical process catalyst, etc., can solve the problem that no report has been made on the synthesis of tricyclic dispiroindole using ketimine, and achieves the effect of efficient and inexpensive manner
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Ethyl (3′R,4′S,5′R)-6″-chloro-4′-(3-chloro-2-fluorophenyl)-2″-oxo-1″,2″-dihydrodispiro[cyclohexane-1,2′-pyrrolidine-3′,3″-indole]-5′-carboxylate
[0228]
[0229]To a mixture of (3E / Z)-6-chloro-3-(3-chloro-2-fluorobenzylidene)-1,3-dihydro-2H-indol-2-one (WO2006 / 091646) (99.9 mg, 0.32 mmol), (R)-BINAP (12.1 mg, 0.019 mmol), and CuOAc (2.0 mg, 0.016 mmol), a solution of cyclohexanone (50.4 μL, 0.49 mmol), glycine ethyl ester (39.6 μL, 0.39 mmol) and triethylamine (6.8 μL, 0.049 mmol) in N,N-dimethylacetamide (2.0 mL) was added under a nitrogen atmosphere, and the resulting mixture was stirred at room temperature for 40 hours. To the reaction mixture, ethyl acetate (2 mL), water (1 mL), and a 20% aqueous ammonium chloride solution (1 mL) were added, and the mixture was vigorously stirred to separate an organic layer. The aqueous layer was subjected to extraction with ethyl acetate twice (2 mL each), and the organic layers were all combined and then washed with water three times (5 mL each). ...
example 2
Ethyl (3′R,4′S,5′R)-6″-chloro-4′-(3-chloro-2-fluorophenyl)-4,4-dimethyl-2″-oxo-1″,2″-dihydrodispiro[cyclohexane-1,2′-pyrrolidine-3′,3″-indole]-5′-carboxylate
[0239]
[0240]To a mixture of (3E / Z)-6-chloro-3-(3-chloro-2-fluorobenzylidene)-1,3-dihydro-2H-indol-2-one (WO2006 / 091646) (98.7 mg), (R)-BINAP (12.1 mg, 0.019 mmol), and CuOAc (2.0 mg, 0.016 mmol), a solution of 4,4-dimethlcyclohexanone (61.4 mg, 0.48 mmol), glycine ethyl ester (39.5 μL, 0.39 mmol) and triethylamine (6.8 μL, 0.049 mmol) in N,N-dimethylacetamide (2.0 mL) was added under a nitrogen atmosphere, and the resulting mixture was stirred at room temperature for 22 hours. To the reaction mixture, ethyl acetate (2 mL), water (1 mL), and a 20% aqueous ammonium chloride solution (1 mL) were added, and the mixture was vigorously stirred to separate an organic layer. The aqueous layer was subjected to extraction with ethyl acetate twice (2 mL each), and the organic layers were all combined and then washed with water three times ...
example 3
Ethyl (3′R,4′S,5′R)-6″-chloro-4′-(2-chloro-3-fluoropyridin-4-yl)-2″-oxo-1″,2″-dihydrodispiro[cyclohexane-1,2′-pyrrolidine-3′,3″-indole]-5′-carboxylate
[0250]
[0251]To a mixture of (3E / Z)-6-chloro-3-[(2-chloro-3-fluoropyridin-4-yl)methylene]-1,3-dihydro-2H-indol-2-one (WO2012 / 121361) (99.2 mg), (R)-BINAP (12.1 mg, 0.019 mmol), and CuOAc (2.0 mg, 0.016 mmol), a solution of cyclohexanone (50.4 μL, 0.49 mmol), glycine ethyl ester (39.6 μL, 0.39 mmol), and triethylamine (6.8 μL, 0.049 mmol) in N,N-dimethylacetamide (2.0 mL) was added under a nitrogen atmosphere, and the resulting mixture was stirred at 0° C. for 18 hours. To the reaction mixture, ethyl acetate (2 mL), water (1 mL), and a 20% aqueous ammonium chloride solution (1 mL) were added, and the mixture was vigorously stirred to separate an organic layer. The aqueous layer was subjected to extraction with ethyl acetate twice (2 mL each), and the organic layers were all combined and then washed with water three times (5 mL each). The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| reaction time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com