Treatment agents for inhibiting HIV and cancer in HIV infected patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Cell Proliferation and Infectivity Assays
[0092]PBLs were isolated from buffy coats of HIV seronegative donors (New York Blood Center). The laboratory-adapted strains HIV BaL and HIV HXB2; primary isolates HIV 92BR020, 92UG031, 93BR029, and 93UG082; and multidrug resistant molecular clone NL4329129-2 were obtained from the NIH AIDS Repository. Primary isolate 2044 was from Paul Clapham (Windeyer Institute), and 1633 and 1638 from the Institute of Human Virology (University of Maryland School of Medicine). Maraviroc, efavirenz, raltegravir, and indinavir were from the NIH AIDS Repository. INK128 was purchased from ApexBio. Proliferation of PBLs was measured by the MTT kit (Roche), following the manufacturer's directions. PBL infectivity assays were performed as described herein.
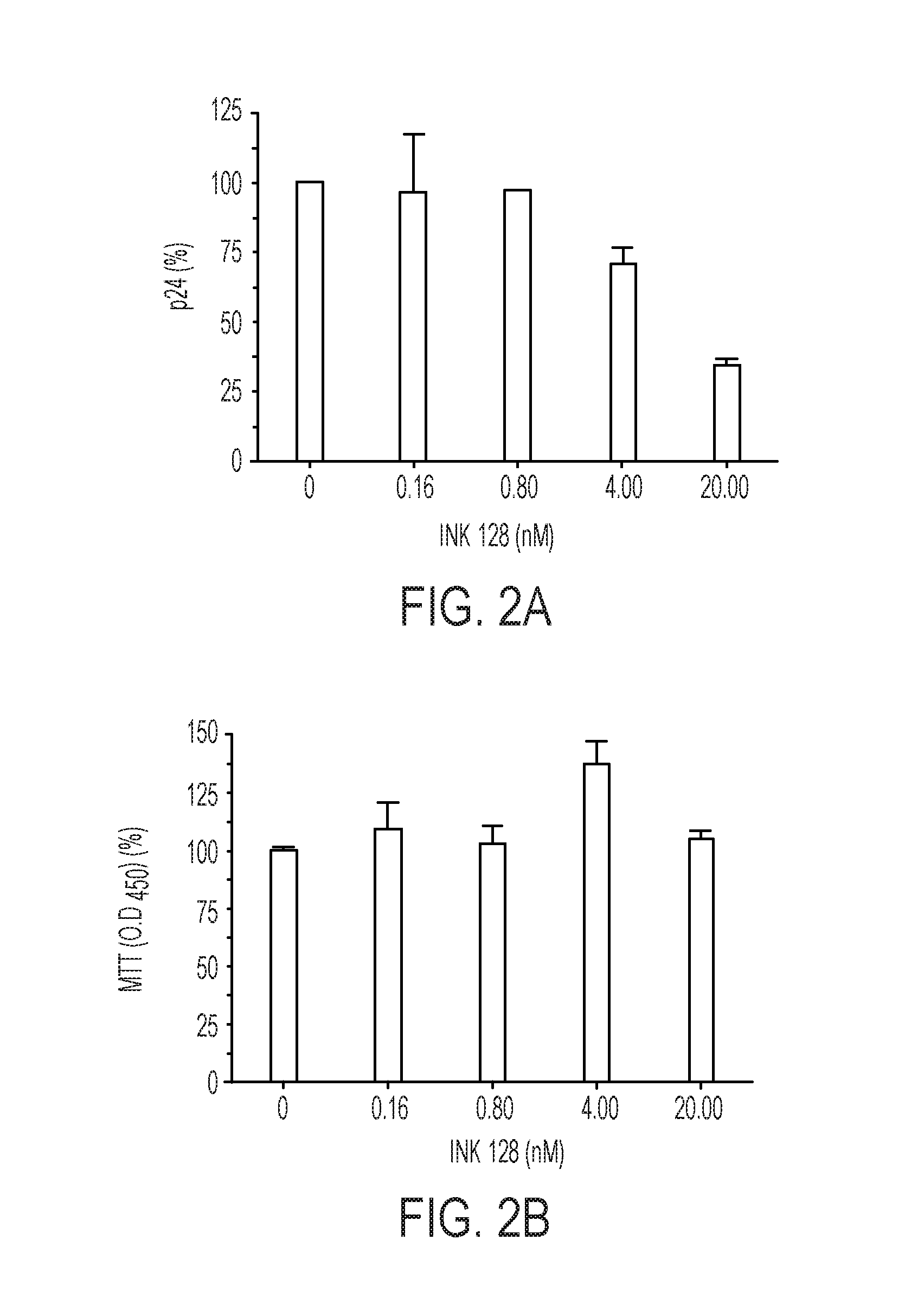
[0093]CD4 cells were isolated from PBL cultures maintained for 7 days in the presence of different concentrations of INK128. Isolation of CD4 cells was done by positive sel...
example 2
INK128 Inhibits R5 and X4 HIV Replication in Primary Cells
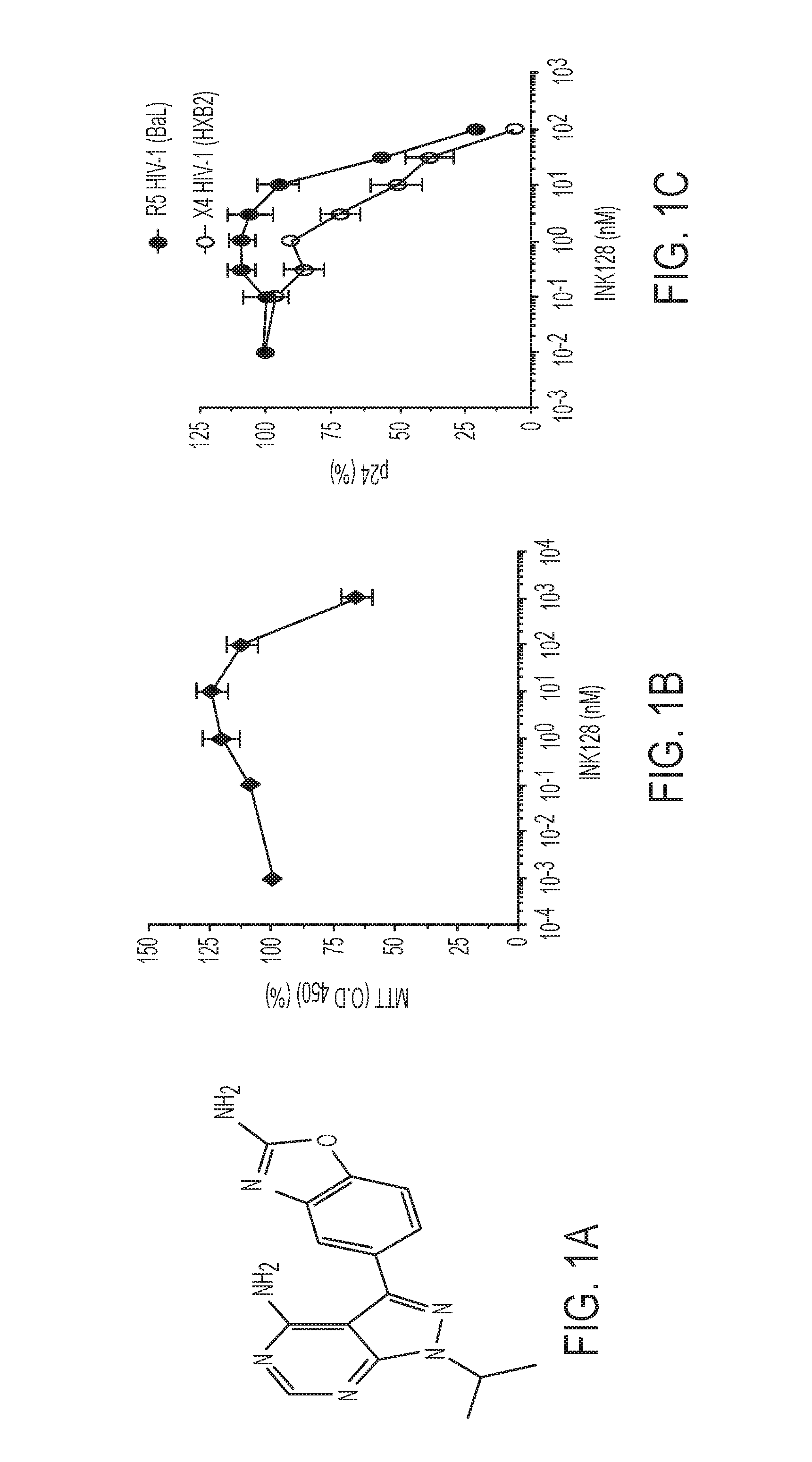
[0104]The chemical structure of INK128 is shown in FIG. 1A. The effect of INK128 was evaluated on proliferation of peripheral blood lymphocytes (PBLs) from four different donors. For each donor, PBLs were activated by treatment with anti-CD3 / CD28 antibodies for 3 days, cultured in the presence of IL-2 and various dilutions of INK128 for 5 days, followed by measurement of cell proliferation by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays (FIG. 1B). INK128 did not inhibit cell proliferation at concentrations of up to 100 nM. Therefore, 100 nM was selected as the highest INK128 concentration in subsequent experiments evaluating antiviral activity.
[0105]The antiviral activity of INK128 was investigated in PBLs infected with CCR5 (R5)-tropic and CXCR4 (X4)-tropic HIV reference strains BaL and HXB2, respectively. In experiments with PBLs from three donors, INK128 inhibited replication of both viruses, ...
example 3
INK128 Inhibits Entry of R5, but not X4, HIV in Primary Lymphocytes
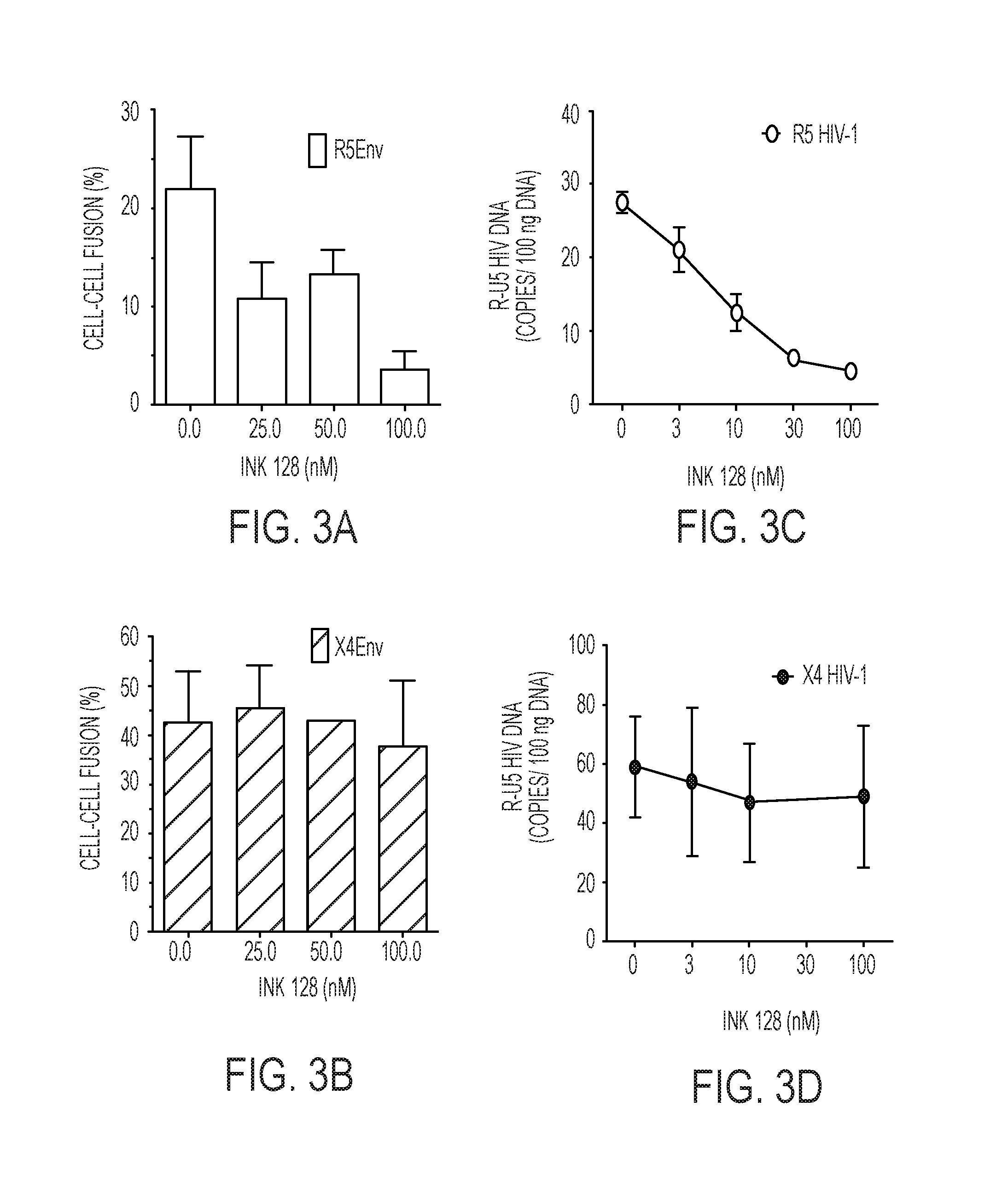
[0107]The mechanism of INK128 inhibition of HIV was evaluated. Because INK128 activity was more potent against R5 than against X4 HIV, it was hypothesized that INK128 affects entry of these viruses differently. To test this, cell-cell fusion assays were performed between 293T cells expressing R5 or X4 HIV envelopes (effectors) and INK128-treated primary CD4+ T cells (targets). In this assay, targets are labeled with the fluorescent dye calcein (green) and effectors with CMTMR (red) before co-culture. Fused cells score positive for both dyes. INK128 inhibited fusion of CD4+ T target cells with R5 HIV JRFL Env, but not with X4 HIV HXB2 Env (FIG. 3A). These data, obtained with CD4 targets from two different donors, suggested inhibition at an early step of the R5, but not X4, HIV lifecycle. Downstream steps in HIV infection were evaluated by measuring early products of reverse transcription and integrated provirus using ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com