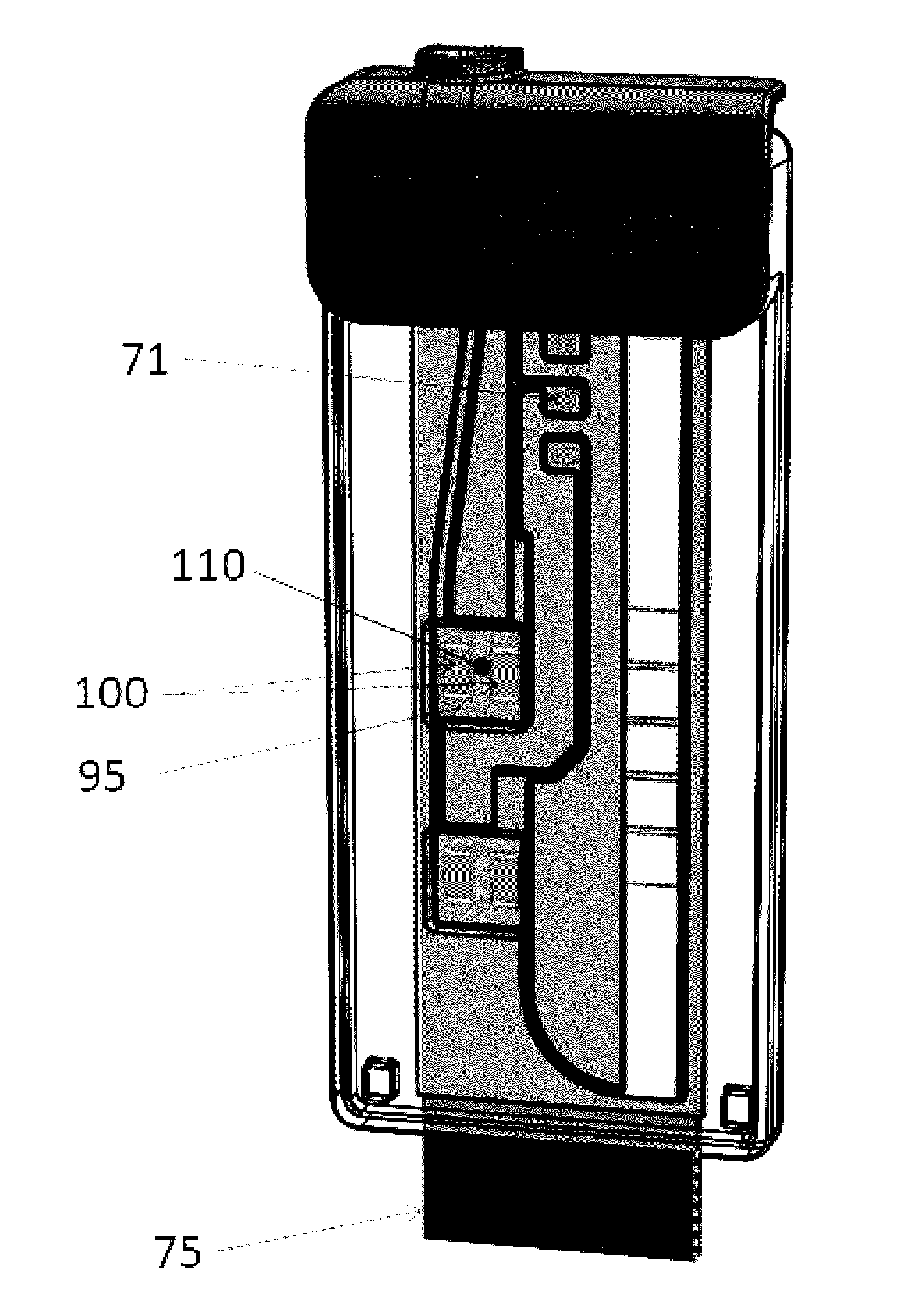
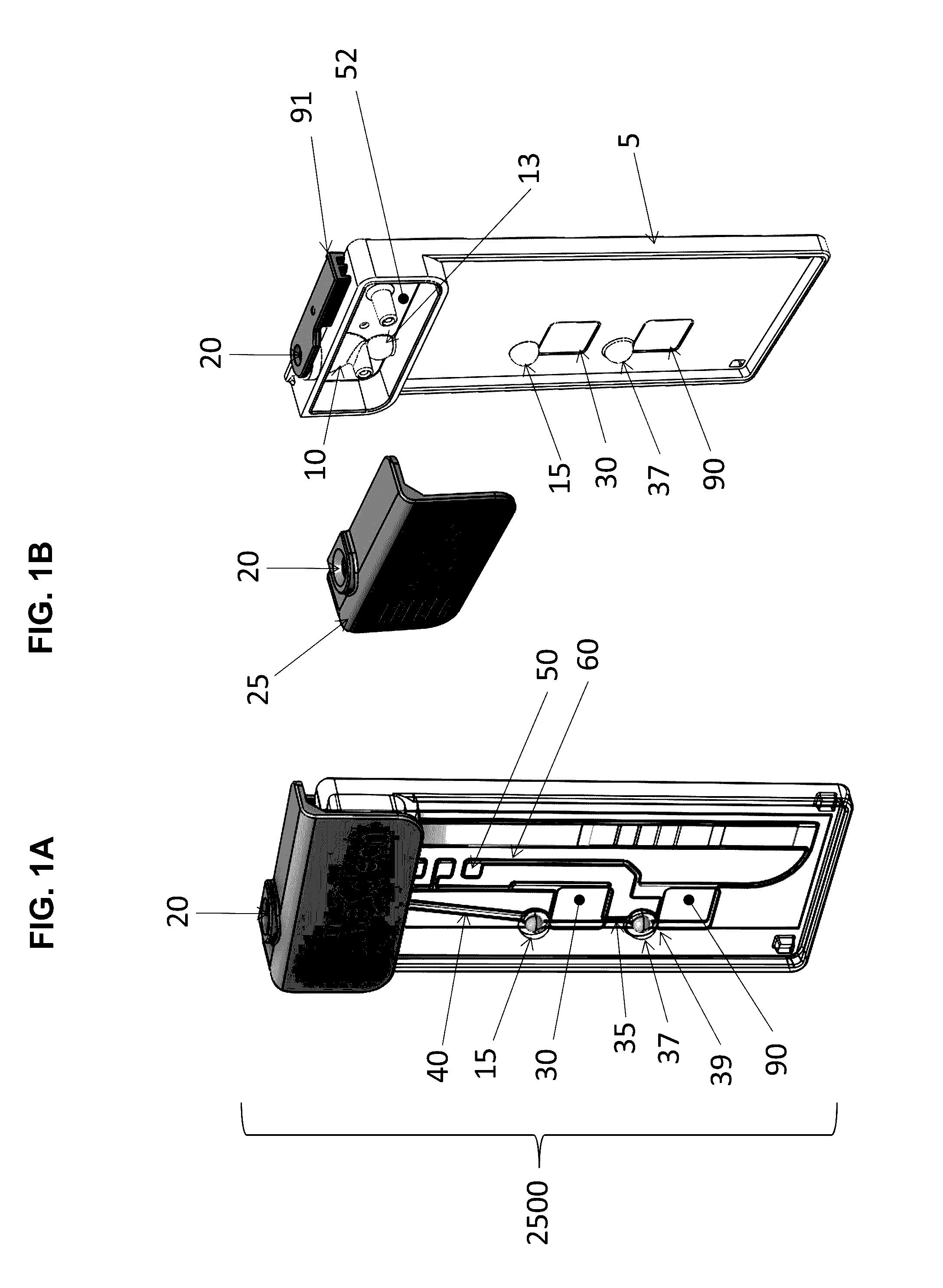
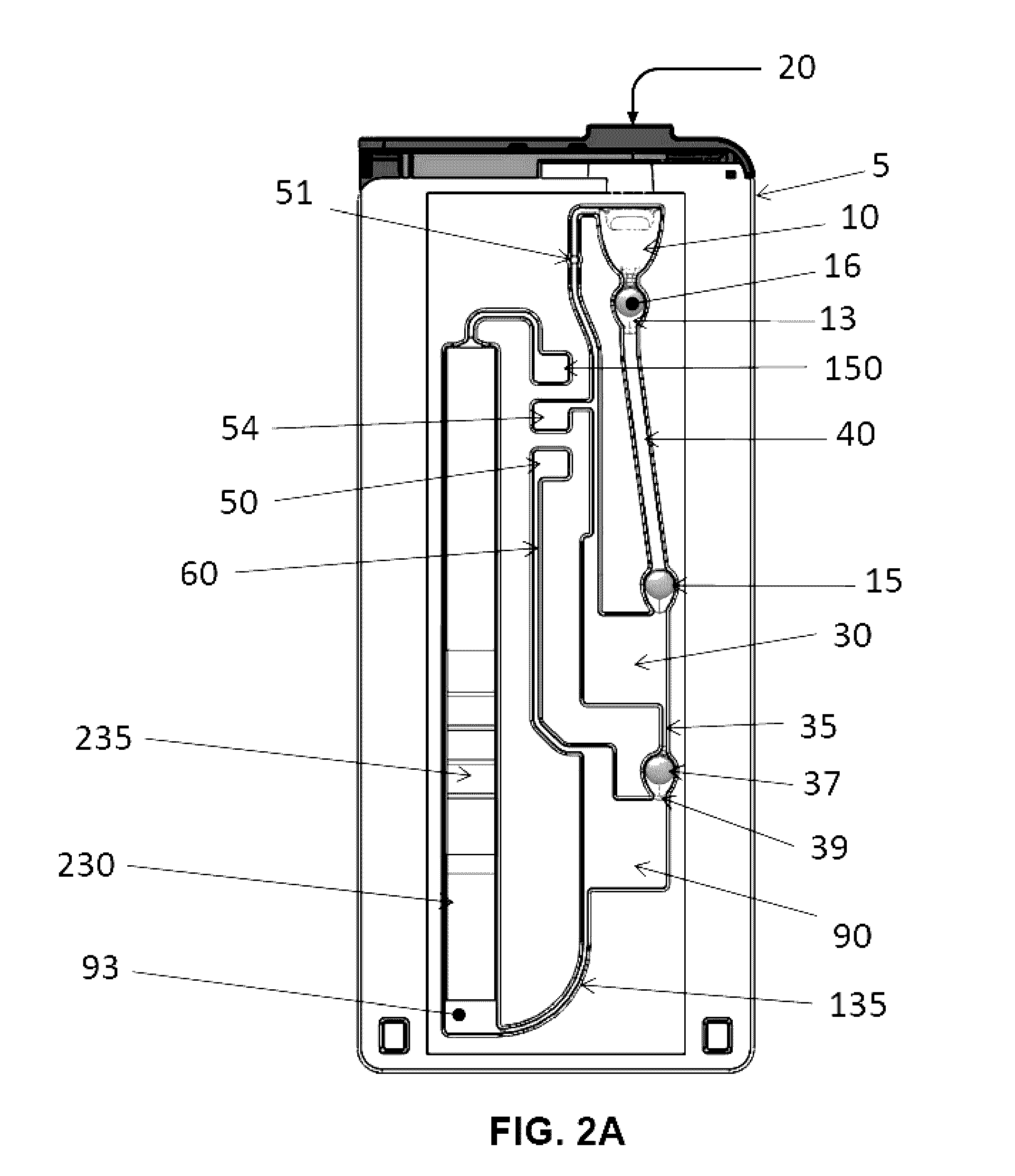
Fluidic Test Cassette
a test cassette and fluidic acid technology, applied in the field of integrated devices, can solve the problems of inconvenient and limited field use of current nucleic acid tests, long turn-around time to obtain the required information, and imposing enormous economic barriers on small clinics, local and state governments and law enforcement agencies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Method of Multiplexed Amplification and Detection of Purified Viral RNA (infA / B) and an Internal Positive Control Virus
[0190]An influenza A and B test cassette was placed into the docking unit. 40 μL of a sample solution was added to the sample port. Sample solutions comprised either purified A / Puerto Rico influenza RNA at a concentration equivalent to 5000 TCID50 / mL, purified B / Brisbane influenza RNA at a concentration equivalent to 500 TCID50 / mL or molecular grade water (no template control sample). Upon entering the sample port, the 40 μL sample comingles with a lyophilized bead as it flows to a first chamber of the test cassette. The lyophilized bead was comprised of MS2 phage viral particles as a positive internal control and DTT. In the first chamber of the cassette the sample was heated to 90° C. for 1 minute to promote viral lysis then cooled to 50° C. prior to opening the vent connected a second chamber. Opening the vent connected to the second chamber allows the sample to ...
example 2
Method of Multiplexed Amplification and Detection of Viral Lysate in Buffer and an Internal Positive Control Virus
[0192]An influenza A and B test cassette was placed into the docking unit. 40 μL of a sample solution was added to the sample port. Sample solutions comprised either A / Puerto Rico influenza virus at a concentration equivalent to 5000 TCID50 / mL, B / Brisbane influenza virus at a concentration equivalent to 500 TCID50 / mL or molecular grade water (no template control sample). Upon entering the sample port, the 40 μL sample comingles with a lyophilized bead as it flows to a first chamber of the test cassette. The lyophilized bead was comprised of MS2 phage viral particles as a positive internal control and DTT. In the first chamber of the cassette the sample was heated to 90° C. for 1 minute to promote viral lysis then cooled to 50° C. prior to opening the vent connected a second chamber. Opening the vent connected to the second chamber allows the sample to flow into the secon...
example 3
Method of Multiplexed Amplification and Detection of Influenza Virus (Purified) Spiked into Negative Clinical Nasal Samples and an Internal Positive Control Virus
[0194]Nasal swab samples collected from human subjects were placed into 3 mL of a 0.025% Triton X-100, 10 mM Tris, pH 8.3 solution and tested for the presence of influenza A and influenza B using an FDA approved real-time RT-PCR test. Samples were confirmed to be negative for influenza A and influenza B prior to use in this study. Confirmed influenza negative nasal sample was spiked with A / Puerto Rico influenza virus at a concentration equivalent to 5000 TCID50 / mL or employed without the addition of virus as a negative control. 40 μL of the resulting spiked or negative control samples were added to the sample port of a influenza A and B test cassette. Upon entering the sample port, the 40 μL sample comingles with a lyophilized bead as it flows to a first chamber of the test cassette. The lyophilized bead was comprised of MS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com