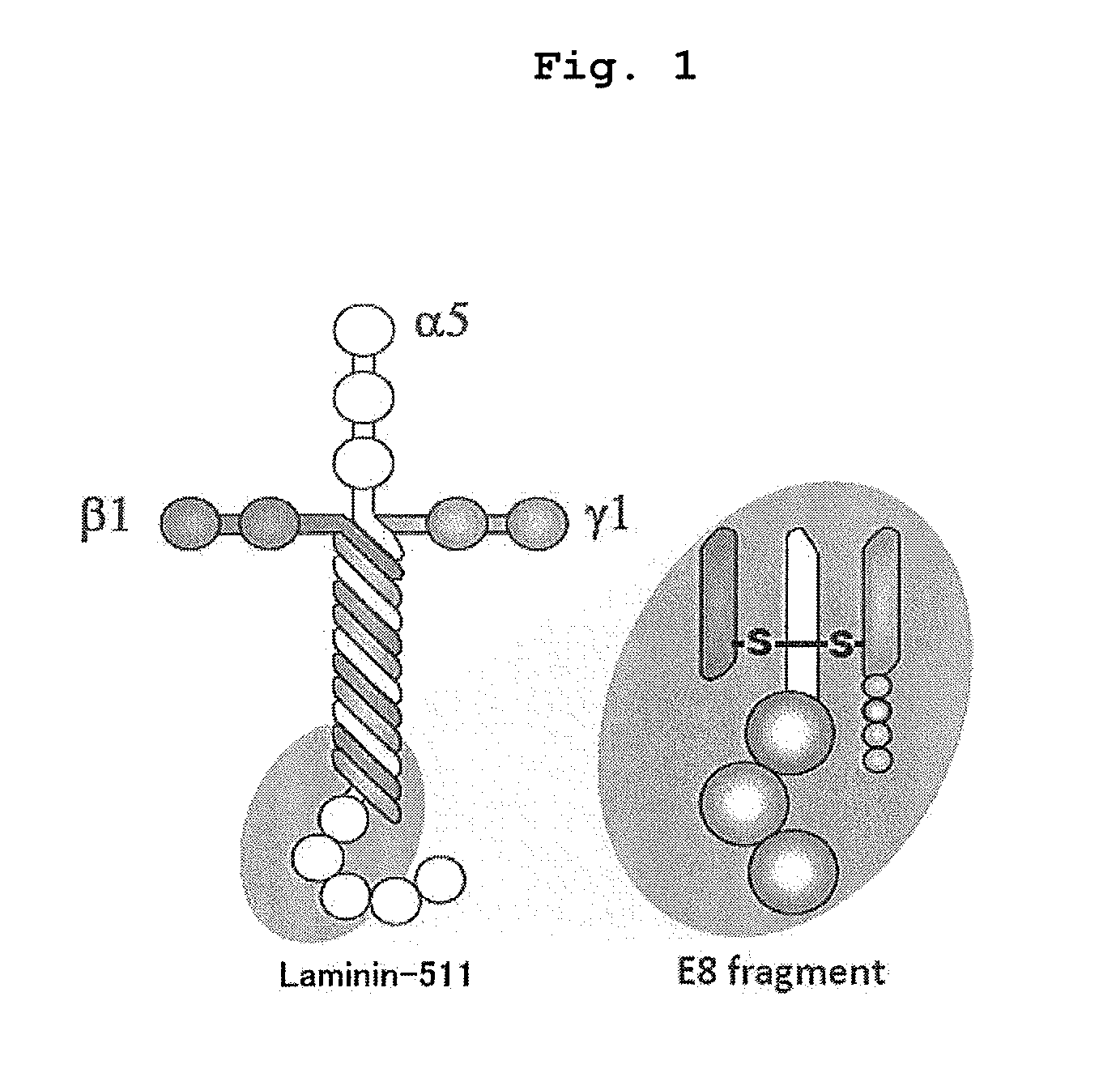
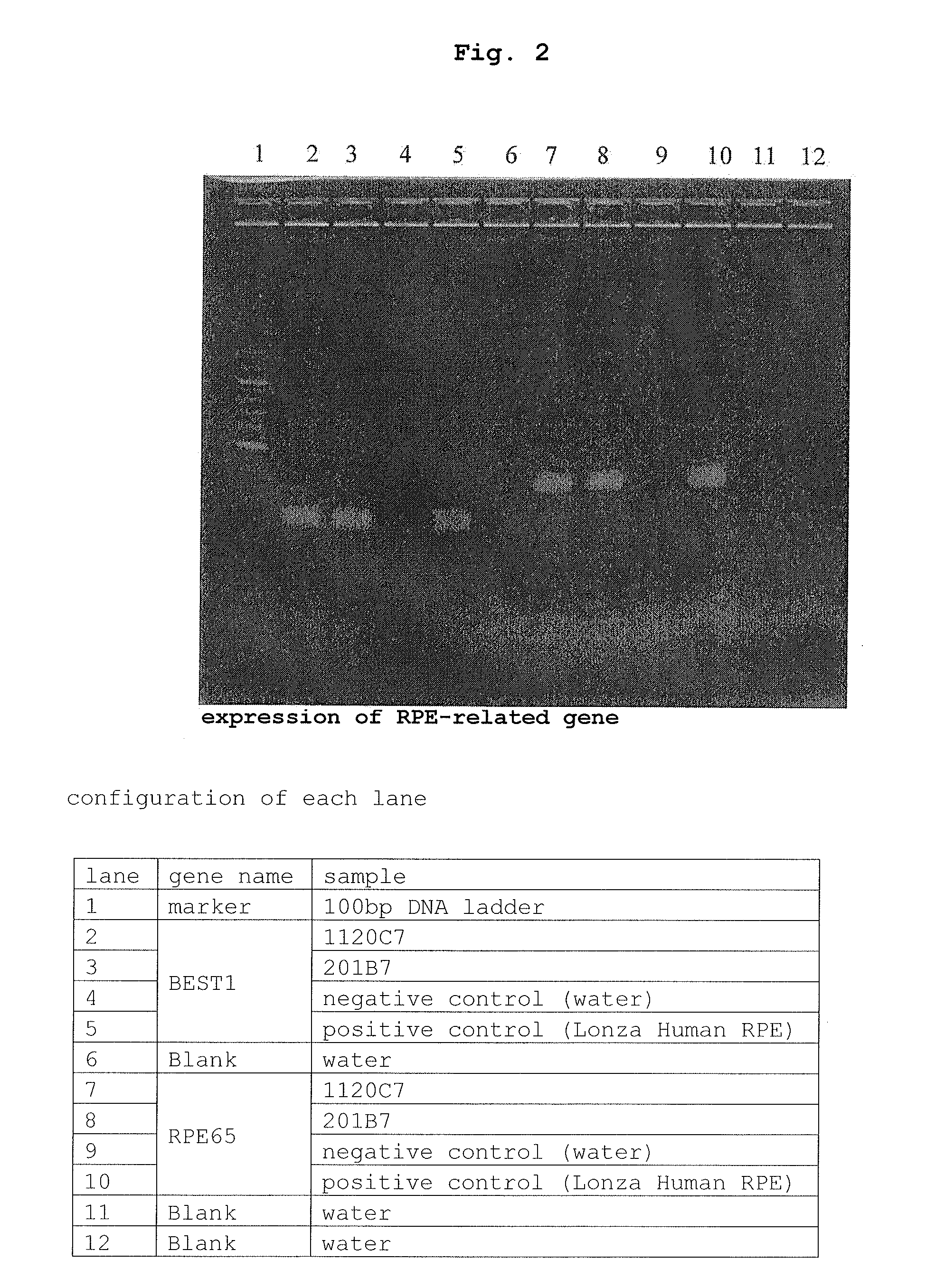
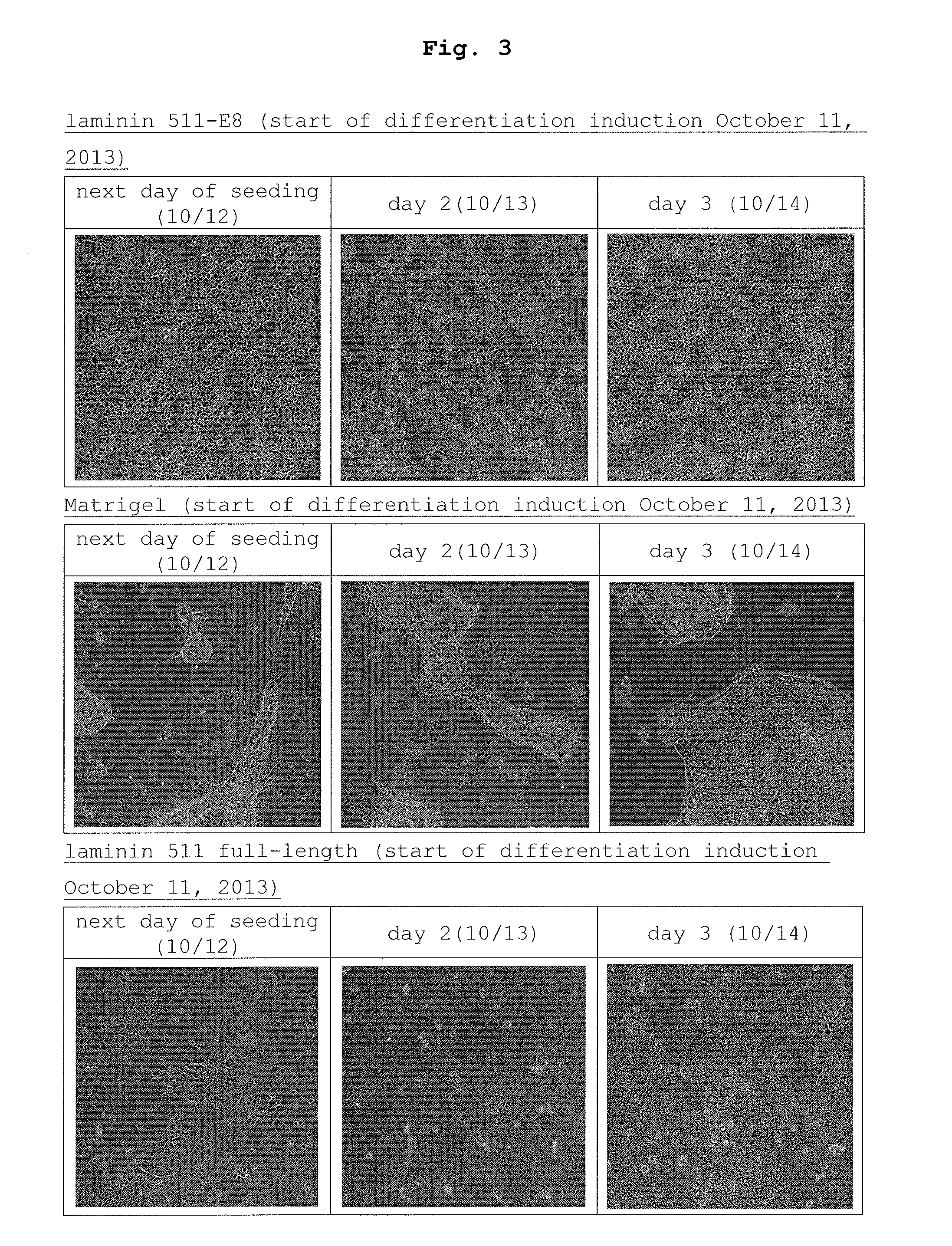
Method of producing retinal pigment epithelial cell
a retinal pigment and epithelial cell technology, applied in cell dissociation methods, cell culture active agents, drug compositions, etc., can solve the problems of easy cell loss, high workload of purification steps, easy cell loss, etc., to improve differentiation induction efficiency, simple and easy purification operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of RPE Cell Derived from iPS Cell
Reagents
[0069]differentiation induction basic medium (GMEM medium (Invitrogen), KSR (Invitrogen), 0.1 mM MEM non-essential amino acid solution (Invitrogen), 1 mM pyruvic acid sodium (SIGMA), 0.1 M 2-mercaptoethanol (Wako Pure Chemical Industries, Ltd.), 100 U / ml penicillin-100 μg / ml streptomycin (Invitrogen))[0070]primary differentiation induction medium (differentiation induction basic medium containing 20% KSR, 10 μM Y-27632 (Wako Pure Chemical Industries, Ltd.), 5 μM SB431542 (SIGMA), 3 μM CKI-7 (SIGMA))[0071]secondary differentiation induction medium (differentiation induction basic medium containing 15% KSR, 10 μM Y-27632 (Wako Pure Chemical Industries, Ltd.), 5 μM SB431542 (SIGMA), 3 μM CKI-7 (SIGMA))[0072]tertiary differentiation induction medium (differentiation induction basic medium containing 10% KSR, 10 μM Y-27632 (Wako Pure Chemical Industries, Ltd.), 5 μM SB431542 (SIGMA), 3 μM CKI-7 (SIGMA))[0073]quaternary differentiation i...
example 2
Production of RPE Cell (Other iPS Cell)
[0079]By a method similar to that of Example 1 except that iPS cells (201B7, provide by Kyoto University) derived from human skin (fibroblast) were used instead of iPS cells (112007, provide by Kyoto University) derived from human peripheral blood (mononuclear cell) were used, pigment cells were obtained.
[0080]As a result, like Example 1, the rate of generation of pigment cells relative to the seeded iPS cells was drastically improved and the differentiation induction efficiency was markedly improved.
example 3
Amplification of RPE Cell Derived from iPS Cell
[0081]The cell population containing pigment cells on Day 47, which underwent adhesion culture in the culture dish in Example 1 and Example 2, was treated with 0.01% Trypsin-0.53 mM EDTA and cell aggregates were detached from the culture dish. Then, adhesion between the cells was detached by mild pipetting. Protease liquid and residual impurities thereof in the cell mixture were removed together with the supernatant by centrifugation, then, unnecessary cells were separated by filtration separation through a cell strainer (DB Falcon Cell Strainer 40 μm Nylon), and a cell population containing RPE cells was recovered (Day 48).
[0082]The obtained cells were seeded in RPE maintenance medium described in Example 1 in the same 5 culture dishes coated with laminin-511 E8 as in Example 1 at 9×106 cells / 9 cm dish, and standing culture was performed until around Day 50 when adhesion of the RPE cell colony was confirmed.
[0083]From Day 51 to Day 71,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| adhesion | aaaaa | aaaaa |
| optical microscope | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com