System and apparatus comprising a multisensor guidewire for use in interventional cardiology
a multisensor guidewire and guidewire technology, applied in the field of system and apparatus comprising a multisensor guidewire for use in interventional cardiology, can solve the problems of increasing the risk of tissue damage, reducing the stiffness of the multisensor guidewire, and difficulty in positioning the sensors, so as to achieve the effect of reducing one or more disadvantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
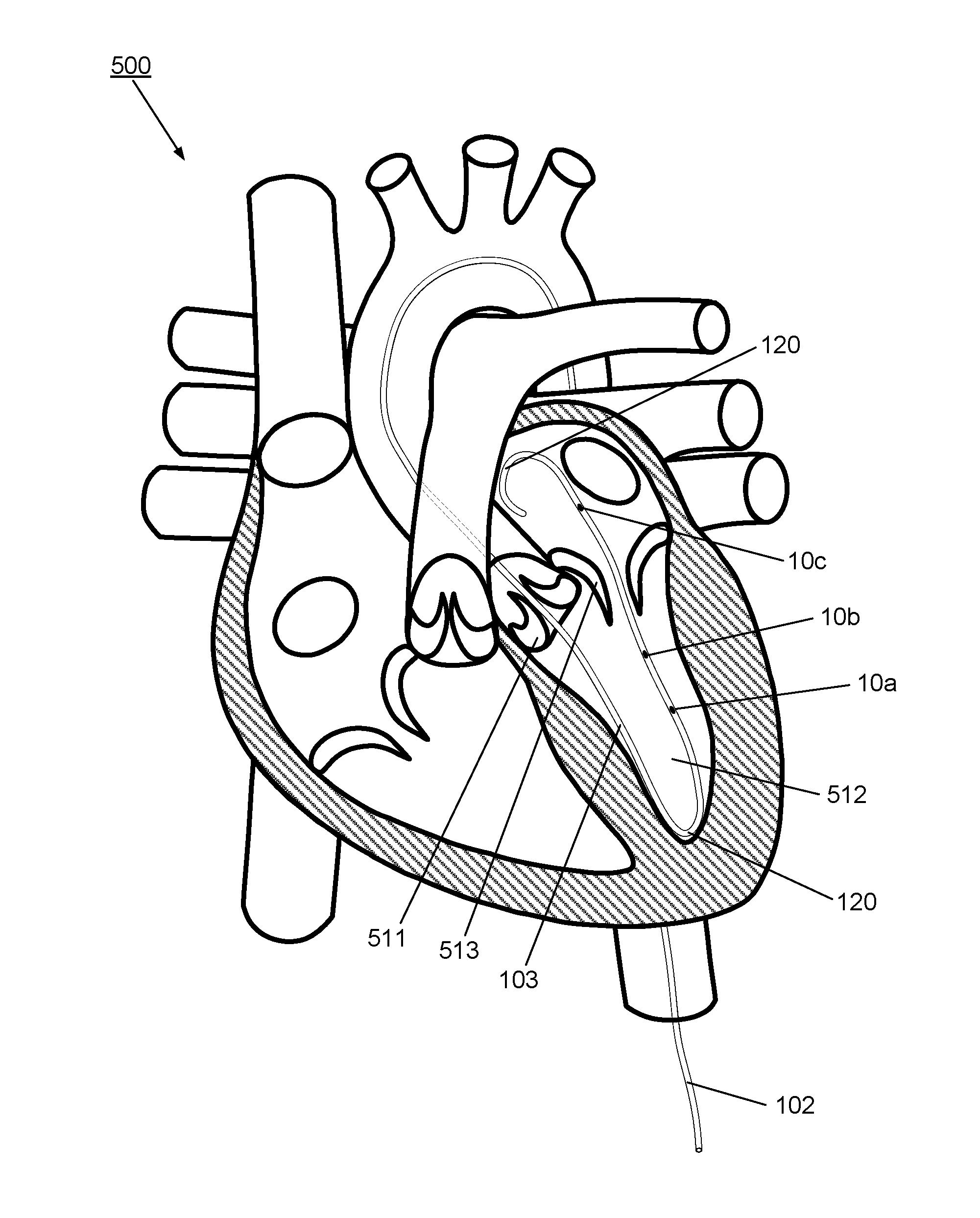
[0090]FIG. 2 illustrates schematically a longitudinal cross-sectional view of the apparatus 100 according to the invention, comprising a multisensor guidewire. The apparatus 100 extends from the optical input / output connector 112 at the proximal end 110 through the proximal part 101 to the distal part 102 which extends to the distal tip 120.
[0091]The distal part 102 takes the form of a multisensor guidewire and comprises components of a conventional guidewire comprising a tubular outer layer in the form of a flexible fine metal coil 35 and an inner mandrel or core wire 31 within the outer coil 35. The outer coil 35 and the core wire 31 each have a diameter and mechanical properties to provide the required flexibility and stiffness to act as a support guidewire for TAVI. Typically, for TAVI, the coil forms a flexible tubular covering layer of the guidewire and has an outside diameter of 0.035 inch or 0.89 mm or less. To provide the appropriate stiffness and other mechanical propertie...
second embodiment
[0104]As illustrated schematically in FIGS. 4B to 4D, assuming the coil 35 has an outside diameter of 0.89 mm (0.035 inch) including any coating, and is formed from 0.002 inch thick coil wire, which would provide a coil with an inside diameter of about 0.787 mm (0.031 inch), then a core wire having a maximum outside diameter of about 0.736 mm (0.029 inch) could be accommodated within, allowing for clearance between the core wire and the coil. Preferably the coil and the core of the guidewire are made from stainless steel having high stiffness and tensile strength, e.g. 304V stainless steel, or other approved types of stainless steel for medical applications. Other biocompatible metal alloys with suitable mechanical characteristics may alternatively be used. Typical medical grade nitinol alloys would not offer sufficient stiffness for a core wire for a multisensor support guidewire for transcatheter valve replacements. On the other hand, medical grade nitinol alloys may provide suffi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com