Novel peptides that bind to types of mhc class ii and their use on diagnosis and treatment
a peptide and mhc class technology, applied in the field of new peptides, can solve the problems of irreversible side effects, risk of side effects caused by a generally suppressed immune defense, and the method of diagnosis does not provide the whole picture of the disease in the individual
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0118]The inventors have identified peptides from human collagen type II, alpha-enolase and vimentin that specifically bind to the variants of MHC that are associated with a risk for developing rheumatoid arthritis and with a presence of antibodies to specific citrullinated proteins. These peptides bind to MHC with genotypes HLA DRB1 0101, 0401, 0404, 0405, 0408 and 1001 as indicated in tables 1, 2 and 3. Interestingly, none of the peptides bind to MHC with genotypes HLA-DR 03 and 0402, genotypes that are not associated with developing rheumatoid arthritis.
[0119]Binding of the peptides to recombinant MHC class II protein of various alleles has been confirmed in an in vitro binding assay and the results are shown in Tables 1, 2 and 3. In table 1, 2 and 3, the listing of the allele in the column for the allele indicates binding. Thus “0401” in the “Binds to 0401” column indicates binding of the peptide to MHC class II of the HLA DRB1 0401 genotype. “no” or no data indicates no binding...
example 2
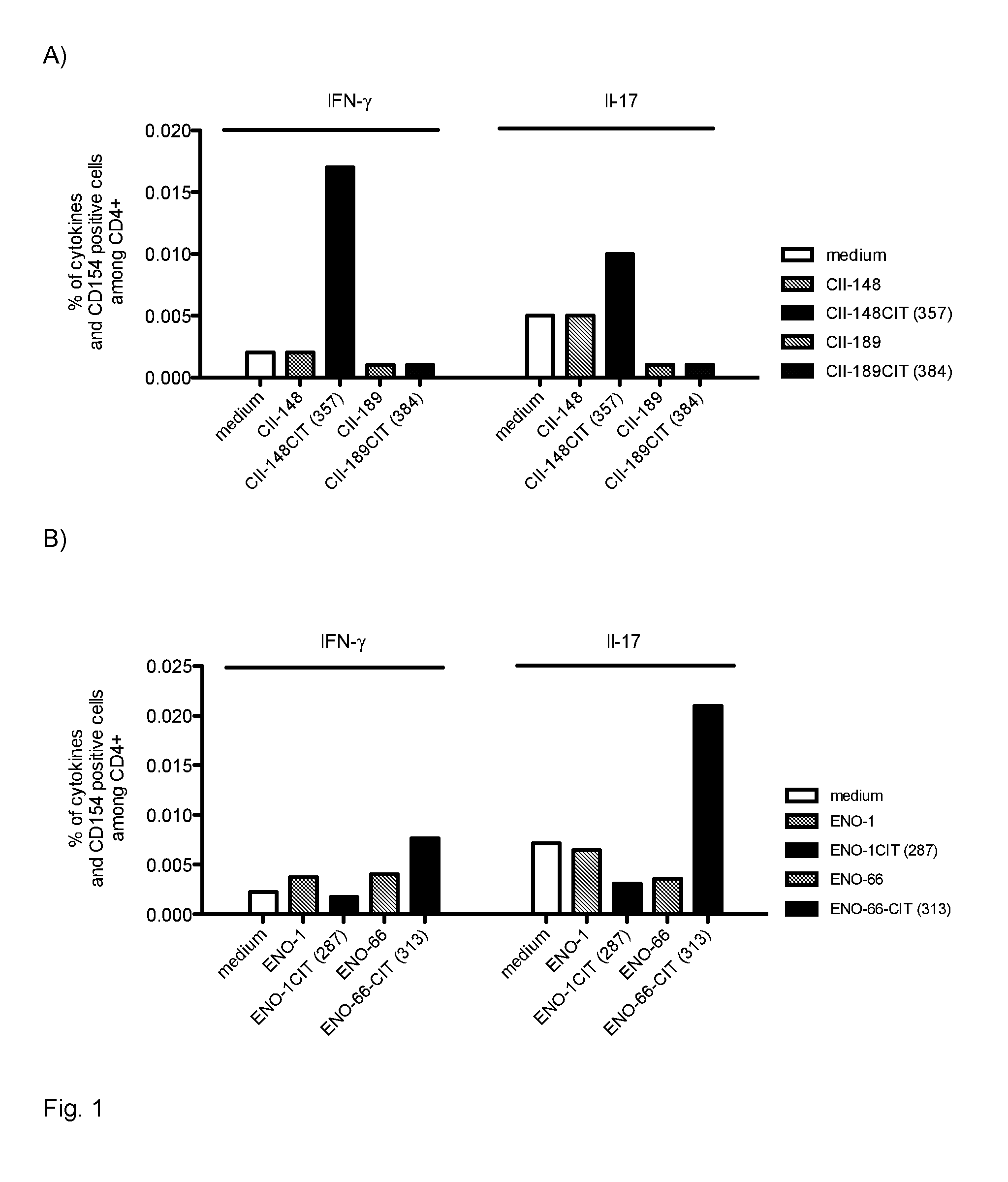
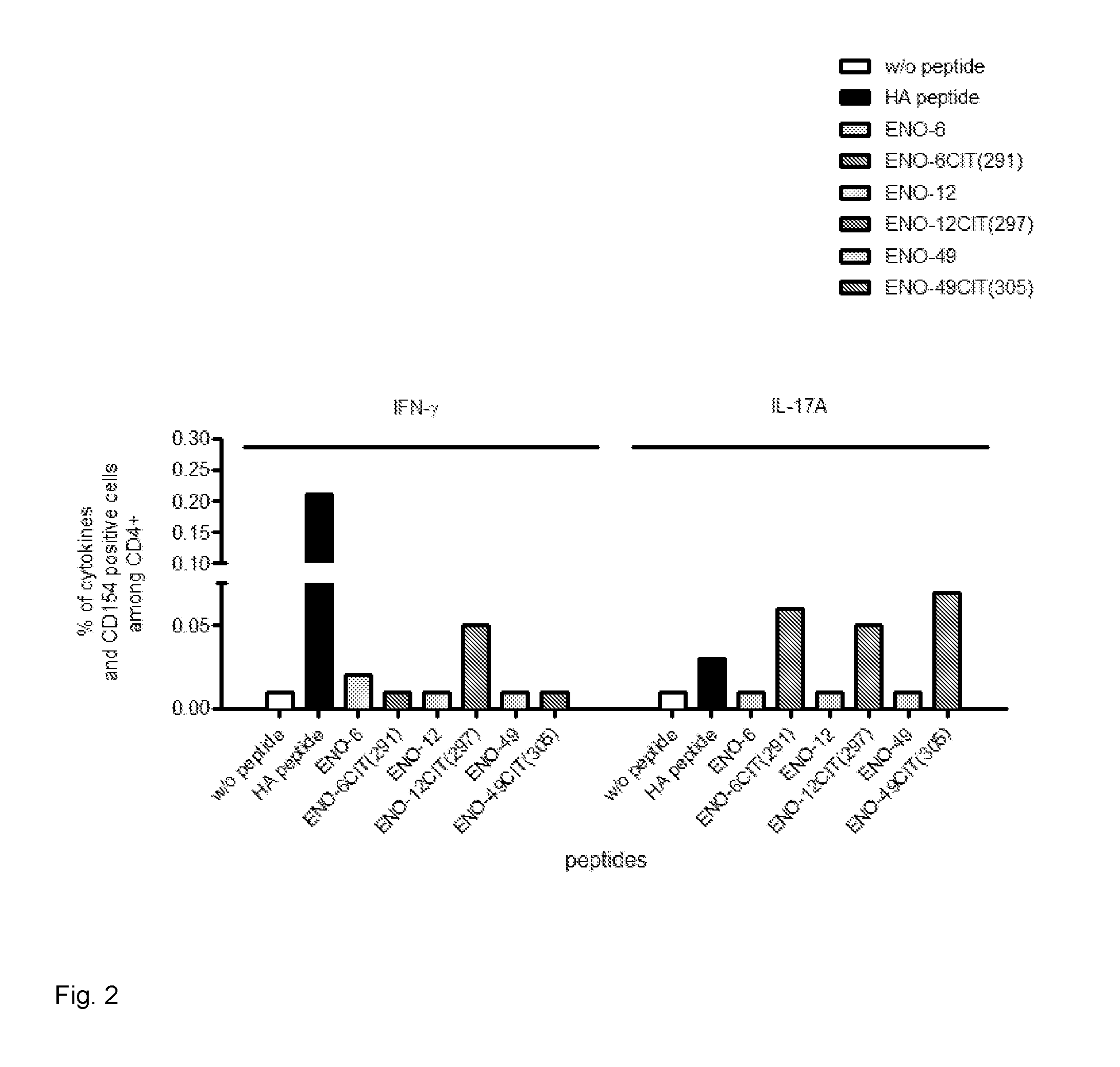
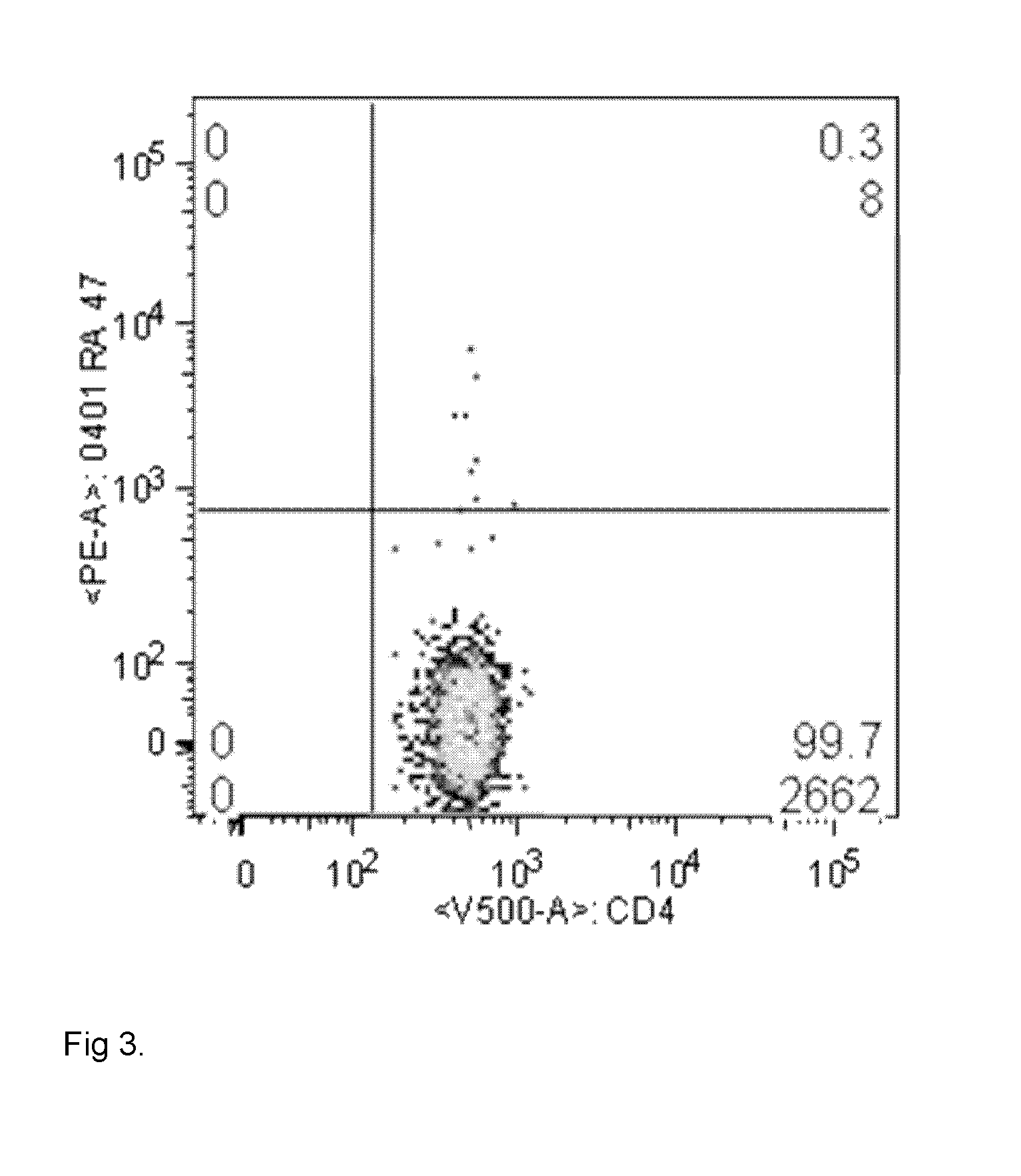
[0122]PBMCs were isolated from several rheumatoid arthritis patients with HLA genotype HLA DRB1-0401. The citrinullated enolase peptide with SEQ ID NO 14 (peptide reference number 287) was added to the cells. As a negative control, cells were incubated without peptide. An influenza peptide was used as a positive control. The peptides were added at concentrations ranging from 5 to 50 ug / ml and after a 5-day incubation in a CO2 incubator, cells were fixed, permeabilized and intracellular cytokine staining were performed. Antibodies for CD3, CD4, viable cells, CD154 (an activation marker) and cytokines IFNgamma, IL-17A and TNF alpha were used for analysis in a multiparameter flow cytometry panel. Cells from all patients exposed to the peptide double stained positive for both CD154 and at least one cytokine, which indicates that activation of T-cells has taken place.
example 3
[0123]PBMCs were isolated from rheumatoid arthritis patients with HLA genotype HLA DRB1-0401. The citrinullated enolase peptide with SEQ ID NO 15 (peptide reference number 289) was added to the cells. As a negative control, cells were incubated without peptide. An influenza peptide was used as a positive control. In addition, the non-citrinullated version of the peptide was used as a negative control. The peptides were added at concentrations ranging from 5 to 50 ug / ml and after a 5-day incubation in a CO2 incubator, cells were fixed, permeabilized and intracellular cytokine staining were performed. Antibodies for CD3, CD4, viable cells, CD154 (an activation marker) and cytokines IFNgamma, IL-17A and TNF alpha were used for analysis in a multiparameter flow cytometry panel. Cells from all patients exposed to the citrinullated peptide double stained positive for both CD154 and at least one cytokine, which indicates that activation of T-cells has taken place. The non-citrinullated pep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com